battery → baterija
Battery a device that converts chemical energy to electrical energy. The process underlying the operation of a battery involves a chemical reaction in which electrons are transferred from one chemical species to another. This process is carried out in two half-reactions, one that involves the loss of electrons and one that involves their gain. The battery is an electrochemical cell divided in two half-cells, and reaction proceeds when these are connected together by an electrically conducting pathway. The passage of electrons from one half-cell to the other corresponds to an electric current. Each half-cell contains an electrode in contact with the reacting species. The electrode which passes electrons into the circuit when battery discharges is called anode and is negative terminal. The electrode which receives electrons is called cathode, and is the battery’s positive terminal. The electrical circuit is completed by an electrolyte, an electrically conducting substance placed between the two electrodes which carriers a flow of charge between them. In wet cells, the electrolyte is a liquid containing dissolved ions, whose motion generates an electrical current; in dry cells the electrolyte is basely solid, for example, a solid with mobile ions or porous solid saturated with an ionic solution.
colligative properties → koligativna svojstva
Colligative properties are properties which affect a solvent based on the number of molecules of solute present such as melting point, boiling point and osmotic pressure.
colloid silver → koloidno srebro
Colloid silver is a bright blue-green powder which dissolved in water gives colloid solution of red colour.
colorimeter → kolorimetar
Colorimeter is an instrument used to measure the strength of colorification in a solution.
colorimetry → kolorimetrija
Colorimetry is a quantitative chemical analysis by colour using a colorimeter.
complete ionic equation → potpuna ionska jednadžba
Complete ionic equation is a balanced equation that describes a reaction occurring in a solution, in which all strong electrolytes are written as dissociated ions.
Beer’s law → Beerov zakon
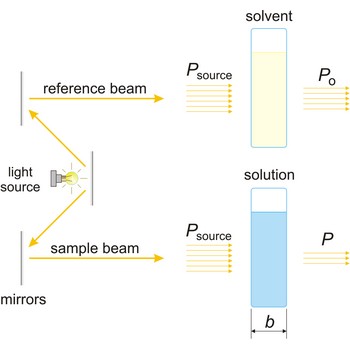
Beer’s law (or Beer-Lambert law) is the functional relationship between the quantity measured in an absorption method (A) and the quantity sought, the analyte concentration (c). As a consequence of interactions between the photons and absorbing particles, the power of the beam is attenuated from Po to P. Beer’s law can be written
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
brass → mjed
Brasses are alloys of copper and zinc (generally 5 % to 40 %). Brass has been known to man since prehistoric times, long before zinc itself was discovered. It was produced by melting copper together with calamine, a zinc ore. Its ductility reaches a maximum with about 30 % zinc and its tensile strength with 45 % although this property varies greatly with the mechanical and heat treatment of the alloy. Typical applications included gears, plumbing ware fittings, adapters, valves and screw machine products. The French horn is a valved brass wind instrument.
Brass may contain small amounts of other alloying elements, such as aluminum, lead, tin, or nickel. Lead can be added as an alloying element resulting in a brass that can be rapidly machined and produces minimal tool wear. Additions of aluminium, iron and manganese to brass improve strength, whilst silicon additions improve wear resistance. Brass containing tin (< 2 % ) is less liable to corrosion in seawater; it is sometimes called naval brass and is used in naval construction.

covering power → prekrivna moć
Covering power is the ability of a plating solution under a set of specified plating conditions to deposit metal on the surfaces of recesses or deep holes. This term suggests an ability to cover, but not necessarily to build up, a uniform coating.
crystallisation → kristalizacija
Crystallisation is process in which the melted substance from a saturated solution turns into solid substance (crystal).
Citing this page:
Generalic, Eni. "Binarna otopina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table