thermometer → termometar
Thermometers are devices for measuring temperature. Linear and volume thermal expansion are macroscopic properties of matter, which can be easily measured, relative to measurements of microscopic properties, on the basis of which, temperature is defined. Thermometers based on thermal expansion are secondary instruments that is, they have to be calibrated in comparison to a standard thermometer. In a thermometer with liquid, mercury or alcohol is placed in a small glass container. If temperature increases, the liquid undergoes volume expansion and rises in a capillary. The level of the raised liquid is the measure of temperature. Mercury thermometers measure temperatures in the temperature range between -39 °C and 300 °C. Alcohol thermometers measure lower temperatures. Bimetal thermometers have a spiral spring, which consists of two metals with different coefficients of linear expansion. When temperature changes, metals undergo different change in length and the consequence twisting of the spring is transferred to a pointer, the deflection of which is the measure of temperature.
Volta, Alessandro → Volta, Alessandro
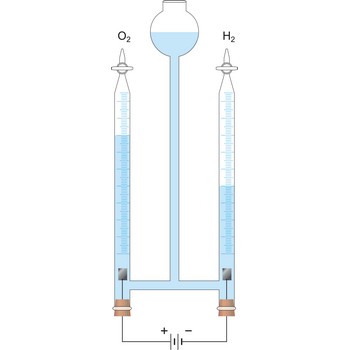
The Italian physicist Alessandro Giuseppe Antonio Anastasio Volta (1745-1827) was the inventor of the voltaic pile, the first electric battery (1800). In 1775 he invented the electrophorus, a device that, once electrically charged by having been rubbed, could transfer charge to other objects. Between 1776 and 1778, Volta discovered and isolated methane gas (CH4). The electrical unit known as the volt was named in his honor.
voltaic pile → Voltin stup
Voltaic pile was the first device that produced a continuous electric current. The first piles constructed by the Italian physicist Alessandro Volta (1745-1827) in 1800 comprised alternating silver and zinc discs separated by cardboard soaked in brine. The pile can be stacked as high as you like, and each layer will increase the voltage by a fixed amount.
zinc → cink
Zinc was discovered by Andreas Marggraf (Germany) in 1746. The origin of the name comes from the German word zink. It is bluish-silver, ductile metal. Reacts with alkalis and acids. Tarnishes in air. Zinc is found in the minerals zinc blende (sphalerite) (ZnS), calamine, franklinite, smithsonite (ZnCO3), willemite and zincite (ZnO). Used to coat other metal (galvanizing) to protect them from rusting. Although some 90 % of the zinc is used for galvanizing steel. Zinc metal is used in the common dry-cell battery. Also used in alloys such as brass, bronze. Zinc compounds are also used in the manufacture of paints, cosmetics, plastics, electronic devices, and other products.
water jet vacuum pump → vodena sisaljka
The water jet vacuum pump or vacuum aspirator is one of the most popular devices that produces vacuum in laboratories. The rapid flow of water through the device creates a vacuum in a side-arm that is connected to a vacuum application such a Buchner flask. The water jet vacuum pump creates a vacuum by means of Venturi effect named after the Italian physicist Giovanni Battista Venturi (1746–1822). The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. Water jet pumps are manufactured of glass, plastic or metal, depending on the conditions in which they are used.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Chitin → Hitin
Chitin is a nitrogen-containing linear polysaccharide of ß(1->4) linked units of N-acetyl-ß-d-glucosamine. The structure of chitin is similar to cellulose except for the replacement hydroxyl group (-OH) at the carbon 2 with an acetyl amine group (–NH–CO–CH3). Chitin is the main component of the exoskeleton, or outer covering of insects, crustaceans, and arachnids. It is also found in the cell walls of certain fungi and algae. After cellulose, chitin is the second most abundant biopolymer in nature. It is insoluble in water, organic solvents, weak acids and lyes.
Citing this page:
Generalic, Eni. "Bible verse about the devil trying to wear the saint out." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table