base-centered orthorhombic lattice → bazno centrirana ortorompska rešetka
Base-centered or side-centered or end-centered monoclinic lattice (orthorhombic-C), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of two parallel sides of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
body-centered cubic lattice → prostorno centrirana kubična rešetka
Body-centered cubic lattice (bcc or cubic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a = b = c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the bcc structures the spheres fill 68 % of the volume. The number of atoms in a unit cell is two (8 × 1/8 + 1 = 2). There are 23 metals that have the bcc lattice.
radioactivity → radioaktivnost
Radioactivity is capability of a spontaneous decay of an atom. In this way a new atom type is formed and radioactive radiation is released. An atom can emit three types of radioactive radiation: positive α-radiation, negative β-radiation and electrically neutral γ-radiation. During radioactive decay one element never emits all types of radiation at the same time.
body-centered orthorhombic lattice → prostorno centrirana ortorompska rešetka
Body-centered orthorhombic lattice (orthorhombic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
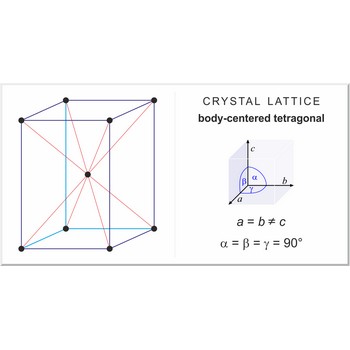
body-centered tetragonal lattice → prostorno centrirana tetragonska rešetka
Body-centered tetragonal lattice (tetragonal-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
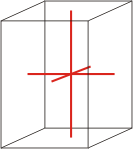 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
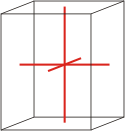 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
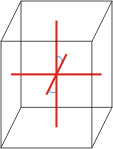 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
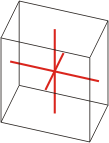 |
a≠b≠c α≠β≠γ≠90° |
cubic crystal system → kubični kristalni sustav
Cubic crystal system is also known as the isometric system. The Isometric crystal system characterizes itself by its three equivalent crystallographic axes perpendicular to each other.
a = b = c
α = β = γ = 90°
disaccharide → disaharid
Disaccharides are compounds in which two monosaccharides are joined by a glycosidic bond. A glycosidic bond to the anomeric carbon can be either α or β. For example, maltose, the disaccharide obtained by enzyme-catalyzed hydrolysis of starch, consists of two D-glucopyranose units joined by a 1,4’-α-glycoside bond. The "prime" superscript indicates that C-4 is not in the same ring as C-1. Unlike the other disaccharides, sucrose is not a reducing sugar and does not exhibit mutarotation because the glycosidic bond is between the anomeric carbon of glucose and the anomeric carbon of fructose.
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
Citing this page:
Generalic, Eni. "Beta-čestica." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table