isoelectric point → izoelektrična točka
Isoelectric point (pI or IEP) is the pH of a solution or dispersion at which the net charge on the molecules or colloidal particles is zero. In electrophoresis there is no motion of the particles in an electric field at the isoelectric point. The net charge (the algebraic sum of all the charged groups present) of any amino acid, peptide or protein, will depend upon the pH of the surrounding aqueous environment. For example, alanine can have a charge of +1, 0, or -1, depending on the pH of the solution in which it is dissolved.
isoleucine → izoleucin
Isoleucine is hydrophobic amino acids with aliphatic side chain. It is one of the three amino acids having branched hydrocarbon side chains. The side chains of these amino acids are not reactive but, these residues are critically important for ligand binding to proteins, and play central roles in protein stability. Isoleucine is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Ile, I
- IUPAC name: 2-amino-3-methylpentanoic acid
- Molecular formula: C6H13NO2
- Molecular weight: 131.12 g/mol
leucine → leucin
Leucine is hydrophobic amino acids with aliphatic side chain. It has one additional methylene group in its side chain compared with valine. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Leucine is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Leu, L
- IUPAC name: 2-amino-4-methylpentanoic acid
- Molecular formula: C6H13NO2
- Molecular weight: 131.17 g/mol
lysine → lizin
Lysine is an electrically charged amino acids with basic side chains. Lysine is a base, as are arginine and histidine. The amino group is highly reactive and often participates in reactions at the active centers of enzymes. Lysine plays an important role in coordinating negatively charged ligands. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Lys, K
- IUPAC name: 2,6-diaminohexanoic acid
- Molecular formula: C6H14N2O2
- Molecular weight: 146.19 g/mol
methionine → metionin
Methionine is neutral amino acids with polar side chains. It is one of the two sulfur-containing amino acids. Methionine is a fairly hydrophobic amino acid and typically found buried within the interior of a protein. It can form stacking interactions with the aromatic moieties of tryptophan, phenylalanine, and tyrosine. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Met, M
- IUPAC name: 2-amino-4-methylsulfanylbutanoic acid
- Molecular formula: C5H11NO2S
- Molecular weight: 149.21 g/mol
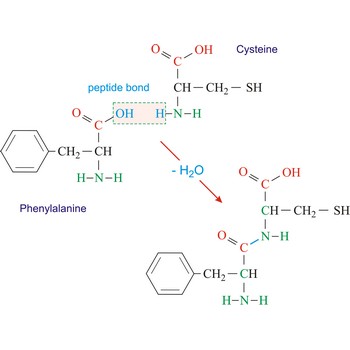
peptide bond → peptidna veza
Peptide bond emerges when two amino acid join in a way that the carbon atom from one connects with the nitrogen atom from the other (creating a C-N bond).
phenylalanine → fenilalanin
Phenylalanine is hydrophobic amino acids with aromatic side chain. It is quite hydrophobic and even the free amino acid is not very soluble in water. Phenylalanine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Phe, F
- IUPAC name: 2-amino-3-phenylpropanoic acid
- Molecular formula: C9H11NO2
- Molecular weight: 165.19 g/mol
polypeptide → polipeptid
Polypeptides are peptides containing ten or more amino acid residues. The properties of a polypeptide are determined by the type and sequence of its constituent amino acids.
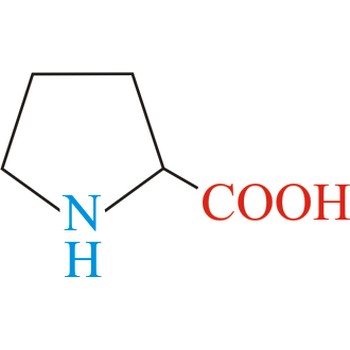
proline → prolin
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
Citing this page:
Generalic, Eni. "Amini." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table