Avogadro constant → Avogadrova konstanta
Avogadro constant (NA or L) is the number of elementary entities in one mole of a substance.
It has the value (6.022 045±0.000 031)×1023 mol-1.
allotropic modification → alotropska modifikacija
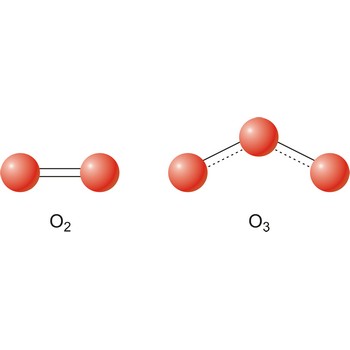
Different substances of the same elementary system are called allotropes or allotropic modifications. In the case of oxygen, there are two allotropic modifications: "normal" dioxygen (O2) and trioxygen (O3) or ozone.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
amount concentration → količinska koncentracija
Amount concentration (also called molar concentration and in older literature molarity) is the amount of a given substance in a stated unit of a mixture, solution, or ore. The common unit is mole per cubic decimetre (moldm−3) or mole per litre (molL-1) sometimes denoted by M.
The concentration of an atom, ion, or molecule in a solution may be symbolised by the use of square brackets, as [Ca2+].
boiling point elevation → povišenje vrelišta
Boiling point elevation is an elevation of the boiling point of a solvent is observed when substances are dissolved in it. The amount by which the boiling point is elevated is proportional to the number of molecules of solute and independent of their nature.
Bronsted acid → Bronstedova kiselina
Brøsted acid is a material that gives up hydrogen ions in a chemical reaction.
Bronsted base → Bronstedova baza
Brønsted base is a material that accepts hydrogen ions in a chemical reaction.
Bronsted-Lowry’s acid-base theory → Bronsted-Lowryeva teorija kiselina i lužina
Brønsted-Lowry’s acid-base theory: Acid is a substance which gives a proton (protondonor) and base is a substance which accepts a proton (protonacceptor).
Citing this page:
Generalic, Eni. "Amfoterna tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table