cross-linking → umrežavanje
Cross-linking is an attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds, as indicated in the schematic diagram
Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin. Cross-linking can be artificially effected, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanisation of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polyethylene by means of high-energy radiation.
Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.
crystallisation → kristalizacija
Crystallisation is process in which the melted substance from a saturated solution turns into solid substance (crystal).
decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
crucible → lončić za žarenje
Crucible is used for heating small amounts of solid in an oven to very high temperatures. Crucibles are usually made out of porcelain, platinum, nickel or iron.
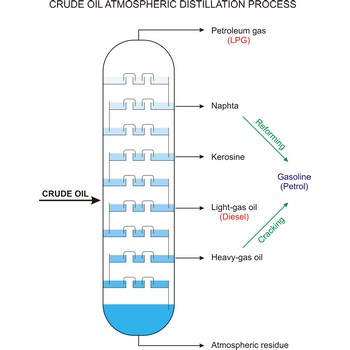
crude oil → sirova nafta
Crude oil (petroleum) is a fossil fuel formed from plant and animal remains many million of years ago. It is occasionally found in springs or pools but is usually drilled from wells beneath the earth’s surface. Crude oil is a mixture of hydrocarbons with small quantities of other chemicals such as sulphur, nitrogen and oxygen. Crude is the raw material which is refined into petrol, heating oil, jet fuel, propane, petrochemicals, and other products.
density → gustoća
In the most common usage, density (ρ) is mass density or mass per unit volume. In Si units it is measured in kg m-3. More commonly, densities are given in kg dm-3.
More generally, it is the amount of some quantity (mass, charge, energy, etc.) divided by a length, area, or volume.
Relative density is the ratio of the density of a substance to the density of some reference substance. For liquids or solids, it is the ratio of the density (usually at 20 °C) to the density of water at 4 °C. This quantity was formerly called specific gravity.
depression of freezing point → snižavanje ledišta
Depression of freezing point of a pure solvent is observed when substances are dissolved in it. The amount by which the freezing point is depressed is proportional to the number of molecules of solute and independent of their nature.
dilute solution → razrijeđena otopina
Dilute solution contains a relatively low concentration of solute.
ebullioscopic constant → ebulioskopska konstanta
Ebullioscopic constant (Eb) is the constant that expresses the amount by which the boiling point Tb of a solvent is raised by a nondissociating solute, through the relation
where b is the molality of the solute.
Citing this page:
Generalic, Eni. "Amfoterna tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table