polarography → polarografija
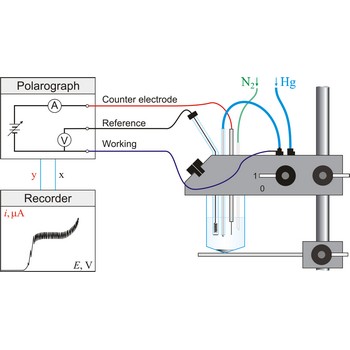
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
polysaccharide → polisaharid
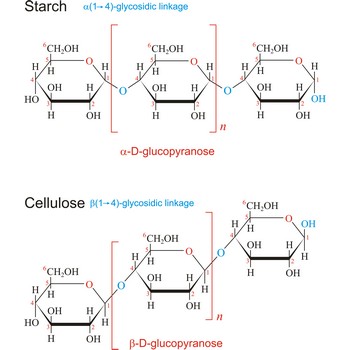
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
reducing agent → redukcijsko sredstvo
Reducing agent may be defined in various ways, depending upon the context in which the phrase is used. In broad terms it is often taken to mean a chemical which can act as an electron donor. Thus, in the reaction:
the zinc is being reduced (gaining electrons) by reaction with the iron cations; the Fe2+ in this instance is acting as a reducing agent.
relative density → relativna gustoća
Relative density (d) is the ratio of the density of a substance to the density of some reference substance. For liquids or solids it is the ratio of the density (usually at 20 °C) to the density of water at 4 °C. Since one must specify the temperature of both the sample and the water to have a precisely defined quantity, the use of this term is now discouraged. This quantity was formerly called specific gravity.
reversible process → reverzibilan proces
Reversible process or reaction is those that can be reversed by an infinitesimally small change in conditions. For example, ice and water coexist at 101 325 Pa and 0 °C; a very slight temperature increase causes the ice to melt; a tiny temperature decrease causes the water to freeze. Melting or freezing under these conditions can be considered reversible.
salt fog test → ispitivanje u slanoj komori
Salt fog test is an accelerated corrosion test in which specimens are exposed to a fine mist of a solution usually containing sodium chloride (typically 5 %). Other contaminants can be added according to desired conditions. It is mainly used to determine the effectiveness of material finishes and protective coatings on materials. Salt-fog testing is also used to determine the effects of salt deposits on the electrical functions of electronic assemblies.
saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
standard deviation → standardna devijacija
Standard deviation (σ) is a measure of the dispersion of a set of data from its mean. Standard deviation is a statistical term that measures the amount of variability or dispersion around an average
Suppose there are many measurements of a quantity presumed to be similar, like the size of peas in a pod. If the number of readings for each size were plotted, a bell-shaped curve would probably result, with a few small and large peas and most clustered around the average size. Around two-thirds of all measurements fall in the range spanned by the standard deviation, a measure of the spread.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
Citing this page:
Generalic, Eni. "Agreement terms and conditions analysed." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table