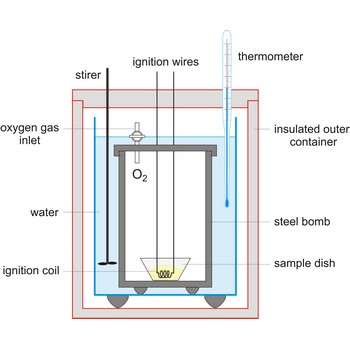
bomb calorimeter → kalorimetrijska bomba
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
deuterium lamp → deuterijeva svjetiljka
Deuterium or hydrogen lamp is used as a source of continuous radiation in UV part of the spectrum.
Devard’s alloy → Devardova legura
Devard’s alloy is an alloy which contains copper, aluminium and zinc (50 % Cu, 45 % Al, 5 % Zn) and is used for nitrate and nitrite reduction in order to obtain ammonia in base media.
electroorganic reaction → elektroorganske reakcije
Electroorganic reaction is an organic reaction produced in an electrolytic cell. Electroorganic reactions are used to synthesise compounds that are difficult to produce by conventional techniques. An example of an electroorganic reaction is Kolbe’s method of synthesising alkanes.
Born-Haber cycle → Born-Haberov kružni proces
Born-Haber cycle is a cycle of reactions used for calculating the lattice energies of ionic crystalline solids. For a compound MX, the lattice energy is the enthalpy of the reaction
The standard enthalpy of formation of the ionic solid is the enthalpy of the reaction
The cycle involves equating this enthalpy (which can be measured) to the sum of the enthalpies of a number of steps proceeding from the elements to the ionic solid. The steps are:
1) Atomization of the metal
2) Atomization of the nonmetal
3) Ionisation of the metal
This is obtained from the ionisation potential.
4) Ionisation of the nonmetal
This is electron affinity.
5) Formation of the ionic solids
Equation of the enthalpies gives
from which ΔHL can be found.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
elementary reaction → elementarna reakcija
Elementary reaction is a reaction that occurs in a single step. Equations for elementary reactions show the actual molecules, atoms, and ions that react on a molecular level.
elution → eluacija
Elution is the process of removing an adsorbed material (adsorbate) from an adsorbent with a liquid (eluent). The solution consisting of the adsorbate dissolved in the eluent is the eluate. Elution is the process used to wash components of a mixture through a chromatography column.
Citing this page:
Generalic, Eni. "Zemljina kora." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table