Results 81–90 of 1083 for Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en
electrodeposition → elektrodepozicija
Electrodeposition is a process of depositing solid materials on an electrode surface using electrolysis. It is a somewhat loosely used term that is applied to many technologies. There are a number of metal deposition technologies. However, not only metals but also different compounds can be electrodeposited. This is used most often for the formation of oxides (such as manganese dioxide and lead dioxide) by anodic oxidation of dissolved salts.
electrodialysis → elektrodijaliza
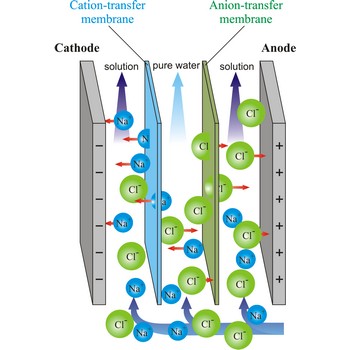
Electrodialysis is a procedure of dialysis accelerated with an electric field. Dialyser is divided into three sections. Solution flows through the middle section, between two semipermeable membranes alternately to positive ions and negative ions. An electrodes are placed in the neighbouring sections. Under the influence of electric field, positive ions will travel towards the cathode (the negative electrode), and negative ions towards the anode (the positive electrode), whereby travelling of ions through the membrane is accelerated. In this way, the feed water is separated into two streams: one of pure water and the other of more concentrated solution.
electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
electrogravimetry → electrogravimetrija
Electrogravimetry is an electroanalytical technique in which the substance to be determined (usually a metal) is deposited out on an electrode which is weighed before and after the experiment. The potential of the electrode must be carefully chosen to ensure that only the metal do be determined will deposit.
ion exchange → ionska izmjena
Ion exchange is a process involving the adsorption of one or several ionic species accompanied by the simultaneous desorption (displacement) of one or more other ionic species.
ionisation energy → energija ionizacije
Ionisation energy is the minimum energy required to remove an electron from an isolated atom or molecule (in its vibrational ground state) in the gaseous phase.
electrolysis → elektroliza
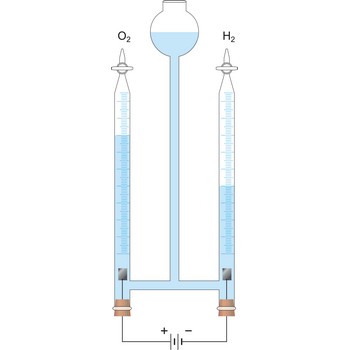
Electrolysis is the decomposition of a substance as a result of passing an electric current between two electrodes immersed in the sample.
electrolytes → elektroliti
Electrolytes are substances which, when melted or dissolved in water, conduct electric current. By melting or dissolving they are dissociated into electrically charged particles (ions) which are able to conduct electric current. By passing of electric current the transfer of matter occurs. Positively charged particles (cations) travel towards the negative pole (the cathode) and negatively charged particles (the anions) travel towards the positive pole (the anode). Liquid metals, in which the conduction is by free electrons, are not usually regarded as electrolytes. Solid conductors of ions, as in the sodium-sulphur cell, are also known as electrolytes. Depending upon how it conducts electric current, matter can be divided into strong electrolytes, weak electrolytes and nonconductors.
electrolytic cell → elektrolitska ćelija
Electrolytic cell is an electrochemical cell that converts electrical energy into chemical energy. The chemical reactions do not occur spontaneously at the electrodes when they are connected through an external circuit. The reaction must be forced by applying an external electric current. It is used to store electrical energy in chemical form (rechargeable battery). It is also used to decompose or produce (synthesise) new chemicals by the application of electrical power. This process is called electrolysis, e.g., water can be decomposed into hydrogen gas and oxygen gas. The free energy change of the overall cell reaction is positive.
electromagnetic radiation spectrum → spektar elektromagnetskog zračenja
Wavelengths of electromagnetic waves can be shown with the help of electromagnetic radiation spectrum. Electromagnetic radiation spectrum is divided into several areas from γ-radiation of very short wavelengths and great energy to radio waves with wavelengths up to 1 000 m. The human eye can only see a narrow part of the electromagnetic spectrum - visible radiation.
Citing this page:
Generalic, Eni. "Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table