Results 411–420 of 1083 for Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en
copolymer → kopolimer
Copolymers are also known as heteropolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as ammonia or water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6.
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers, e.g. acrylonitrile-butadiene-styrene copolymer (ABS). Varying the proportions of the component monomers can preselect its properties.
copper → bakar
Copper has been known since ancient times. The origin of the name comes from the Latin word cuprum meaning the island of Cyprus famed for its copper mines. It is malleable, ductile, reddish-brown metal. Resistant to air and water. Exposed surfaces form greenish carbonate film. Pure copper occurs rarely in nature. Usually found in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Most often used as an electrical conductor. Also used in the manufacture of water pipes. Its alloys are used in jewellery and for coins.
corrosion → korozija
Corrosion is a harmful and undesirable construction material consumption by the chemical activity of its surroundings. Corrosion concept refers to metal and nonmetal construction materials, but it is usually used for metals, Corrosion of metal, according to the mechanism process, is divided into chemical (corrosion in nonelectrolytes) and electrochemical (corrosion in electrolytes).
Chemical corrosion appears by direct action of molecule of some element or compound on metal, thus directly creating corrosion products.
Electrochemical corrosion of metals occurs in electrolytes, so reduction of metal atom into free cation appears which by secondary processes gives molecules of compound which are considered a corrosion product.
Coulomb’s law → Coulombov zakon
Coulomb’s law is the statement that the force F between two electrical charges q1 and q2 separated by a distance r is
where εo is the permittivity of a vacuum, equal to
coulometer → kulometar
Coulometer is a type of electrolysis cell which is used for measuring the quantity of one element released during electrolysis.
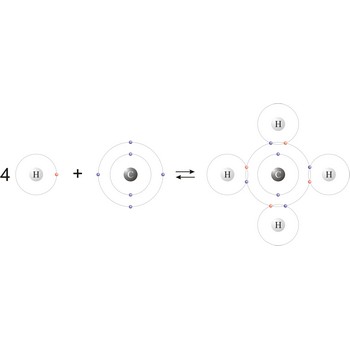
covalent bond → kovalentna veza
Covalent bond is a chemical bond between two atoms whose stability results from the sharing of two electrons, one from each atom (H· + ·H = H:H or H-H).
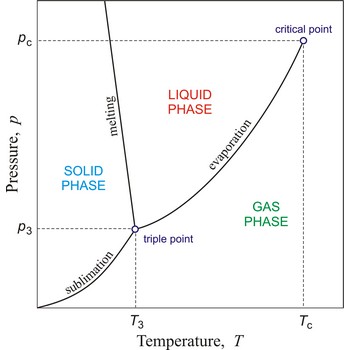
critical point → kritična točka
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
cross-linking → umrežavanje
Cross-linking is an attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds, as indicated in the schematic diagram
Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin. Cross-linking can be artificially effected, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanisation of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polyethylene by means of high-energy radiation.
Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.
Citing this page:
Generalic, Eni. "Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table