Results 401–410 of 1083 for Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
colloid ion → koloidni ion
Colloid ions emerge when colloid particles adsorb certain type of ion from solution and thus become charged with the same charge. The charge can also originate form a chemical reaction of colloid particle’s surface. Colloid ions formed by absorption of silver chloride particle can be show as follows:
Adsorbed layer is monomolecular (one molecule thick) and which type of ion will be formed depends upon which ions are present in a greater number in the solution in. Because of this colloid particles are charged with the same charge, mutual repelling occurs, and the colloid solution becomes stable. Colloid charge can be determined by electrophoresis.
colloid mill → koloidni mlin
Colloid mills are machines used to grind aggregates into very fine particles or to apply very high shearing within a fluid to produce colloid suspensions or emulsions in which the particle sizes are less than 1 micrometer. One type of colloid mill is called a disc mill, in which a mixture of a solid and liquid (or two liquids) is passed between two discs a small distance apart, which rotate very rapidly relative to each other. Applications of colloid mills occur in food processing, in paint manufacture, and in the pharmaceutical industry.
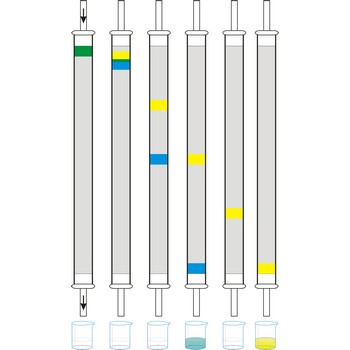
column chromatography → kromatografija u koloni
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
combustible → zapaljiv
The term combustible is often used to describe any material which will burn. However, conditions at which combustion will occur must be defined more accurately, for example a combustible liquid is 37.8 °C but below 93.3 °C. This allows a distinction to be made between combustible materials, which are fairly difficult to ignite, and flammable or highly flammable ones, which are far easier to ignite.
complexometry → kompleksometrija
Complexometry is a volumetric analytic method which is based on titration of metal ion solutions with a substance that, combined with metal ions yields complex compounds, e.g. EDTA. The stoichiometric ratio of EDTA-metal in complexometric analyses is always 1:1, whatever the valency of the metal
conformation → konformacija
Conformation is one of the very large numbers of possible spatial arrangements of atoms that can be interconverted by rotation about a single bond in a molecule. The conformation of a molecule is not fixed, though one or another shape may be more likely to occur. There are two extreme cases:
Staggered conformation (antiperiplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as far apart as possible from the atoms on an adjacent carbon.
Eclipsed conformation (syn-periplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as close as possible to the atoms on an adjacent carbon.
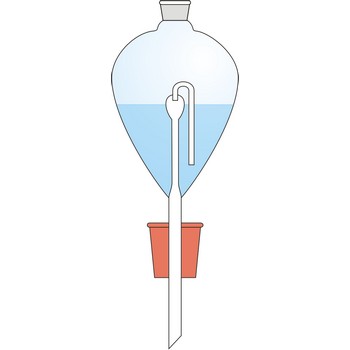
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
control rod → kontrolna šipka
Control rods are rods in a nuclear reactor composed of substances that absorb neutrons (cadmium, hafnium, boron, etc.). These rods regulate the power level of the reactor.
Citing this page:
Generalic, Eni. "Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table