Results 391–400 of 1083 for Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en
chlorophyll → klorofil
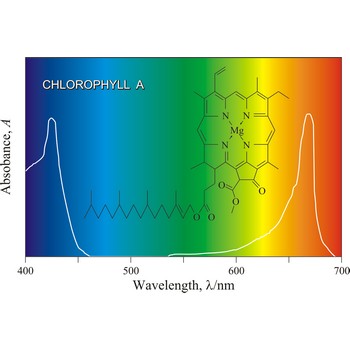
Chlorophyll is a green pigment present in green plants and cyanobacteria. Chlorophyll is essential in the transformation of light energy to chemical energy in photosynthesis. Chlorophyll absorbs light mostly in the blue and red ends of the visible spectrum, and very little in the green wavelengths. That green light is reflected, giving us the leaf colour we see.
chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
chromatography → kromatografija
Chromatography is a method of separation of the components of a sample in which the components are distributed between two phases, one of which is stationary while the other moves. In gas chromatography, the gas moves over a liquid or solid stationary phase. In liquid chromatography, the liquid mixture moves through another liquid, a solid, or a gel. The mechanism of separation of components may be adsorption, differential solubility, ion-exchange, permeation, or other mechanisms.
chromium → krom
Chromium was discovered by Louis-Nicholas Vauquelin (France) in 1797. The origin of the name comes from the Greek word chroma meaning colour. It is very hard, crystalline, steel-grey metal. The pure metal has a blue-white colour. It is hard, brittle and corrosion-resistant at normal temperatures. Hexavalent compounds toxic by skin contact. The most important chromium mineral is chromite [Fe,Mg(CrO4)]. Produced commercially by heating its ore in the presence of silicon or aluminium. Used to make stainless steel. It gives the colour to rubies and emeralds. Iron-nickel-chromium alloys in various percentages yield an incredible variety of the most important metals in modern technology.
clay triangle → trokut za žarenje
Clay triangle is a piece of laboratory equipment used in the process of heating substances by a Bunsen burner (e.g. to support a crucible when it’s being heated).
close-packed structure → gusta slagalina
Close packing is the packing of spheres so as to occupy the minimum amount of space. The name close packed refers to the packing efficiency of 74.05 %. There are two types of close packing: hexagonal and cubic. One layer, with atoms centered on sites labeled a. Two layers, with the atoms of the second layer centered on sites labeled b. The third layer can be placed on the sites labeled c (giving cubic close-packing) or over those marked a (giving hexagonal close-packing).
cobalt → kobalt
Cobalt was discovered by Georg Brandt (Germany) in 1735. The origin of the name comes from the German word kobald meaning goblin or evil spirit. It is hard, ductile, lustrous bluish-grey metal. Surfaces stable in air. Reacts over time with dilute acids. It has remarkable magnetic properties. Cobalt occurs in compounds with arsenic and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a by-product of refining nickel, copper and iron. Used in many hard alloys; for magnets, ceramics and special glasses. Radioactive cobalt-60 is used in cancer therapy.
coenzyme a → koenzim a
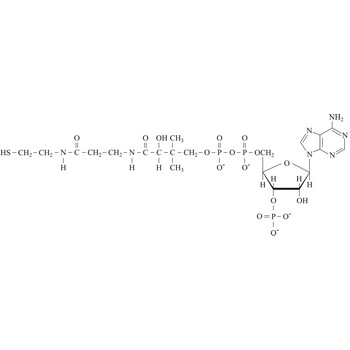
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
Citing this page:
Generalic, Eni. "Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table