Results 381–390 of 1083 for Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en
cetane number → cetanski broj
Cetane number is a measure of the ignition quality of diesel fuel. It denotes the volume fraction of cetane (C16H34) in a combustible mixture (containing cetane and 1-methylnapthalene) whose ignition characteristics match those of the diesel fuel being tested. Cetane is a collection of un-branched open chain alkane molecule that ignites very easily under compression, so it was assigned a cetane number of 100, while alpha-methyl naphthalene was assigned a cetane number of 0.
chain reaction → lančana reakcija
Chain reaction is a reaction done in three steps: initiation in which usually radicals are made which react with other molecules in stage of propagation and, when all reactants are spent, it ends by termination when all available radicals are completely spent.
Nuclear chain reaction refers to a process in which neutrons released in fission produce an additional fission in at least one further nucleus. Nuclear power plants operate by precisely controlling the rate at which nuclear reactions occur. On the other hand, nuclear weapons are specifically engineered to produce a reaction that is so fast and intense it cannot be controlled after it has started.
change of state → promjena stanja
Change of state is a physical change which appears when a substance crosses from one state into another. This usually happens because of the change of energy of particles provoked by heating or cooling.
Charles’ law → Charlesov zakon
The volume of a fixed mass of gas at a constant pressure expand by the constant fraction of its volume at 0 °C. For each Celsius or kelvin degree its temperature is raised. For any ideal gas fraction it is approximately 1/273. This can be expressed by the equation
were V° is the volume at 0°C and V is its volume at t°C.
This is equivalent to the statement that the volume of a fixed mass of gas at a constant pressure is proportional to its thermodynamic temperature
This law also know as Gay-Lussac’s law.
An equation similar to the one given above applies to pressures for ideal gases:
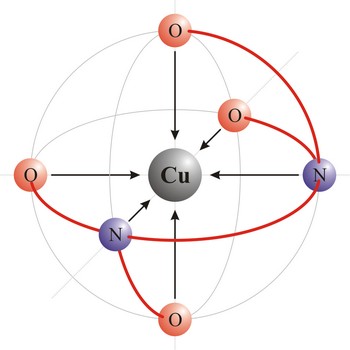
chelate → kelat
Chelate is a compound characterized by the presence of bonds from two or more bonding sites within the same ligand to a central metal atom. For example, copper complexes with EDTA to form a chelate. Chelate complexes are more stable than complexes with the corresponding monodentate ligands.
chemical compound formula → formula kemijskog spoja
Chemical elements are represented by their symbols, and chemical compounds are represented by a group of symbols of those elements from which the compound is composed. That group of symbols, which shows which atoms and in which number relation they are present in certain compound is called a chemical compound formula.
In a formula chemical symbols show which element is present in a certain compound, and its index shows how much of that element there is in a certain compound. From sulphuric acid formula H2SO4 we can see that one molecule of sulphuric acid consists of two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
chemical potential → kemijski potencijal
For a mixture of substances, the chemical potential of constituent B (μB) is defined as the partial derivative of the Gibbs energy G with respect to the amount (number of moles) of B, with temperature, pressure, and amounts of all other constituents held constant.
Also called partial molar Gibbs energy. Components are in equilibrium if their chemical potentials are equal.
chemical weapons → kemijsko oružje
The Chemical Weapons Convention, article 2, paragraph 1 defines chemical weapons thus:
Chemical weapons means the following, together or separately:
(a) Toxic chemicals and their precursors, except where intended for purposes not prohibited under this Convention, as long as the types and quantities are consistent with such purposes;
(b) Munitions and devices, specifically designed to cause death or other harm through the toxic properties of those toxic chemicals specified in subparagraph (a), which would be released as a result of the employment of such munitions and devices;
(c) Any equipment specifically designed for use directly in connection with the employment of munitions and devices specified in subparagraph (b).
chiral molecule → kiralne molekule
Chiral molecule is a molecule which cannot be superimposed on its mirror image. A common example is an organic molecule containing a carbon atom to which four different atoms or groups are attached. Such molecules exhibit optical activity, i.e., they rotate the plane of a polarised light beam.
chlorine → klor
Chlorine was discovered by Carl William Scheele (Sweden) in 1774. The origin of the name comes from the Greek word chloros meaning pale green. It is greenish-yellow, disagreeable gas with irritating odour. Gas is toxic and severe irritant by contact or inhalation. Never found in free form in nature. Commercial quantities of chlorine are produced by electrolysis of aqueous sodium chloride (NaCl) from seawater or brine from salt mines. Used in water purification, bleaches, acids and many, many other compounds such as chlorofluorocarbons (CFC).
Citing this page:
Generalic, Eni. "Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table