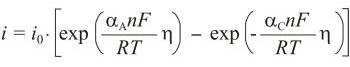Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
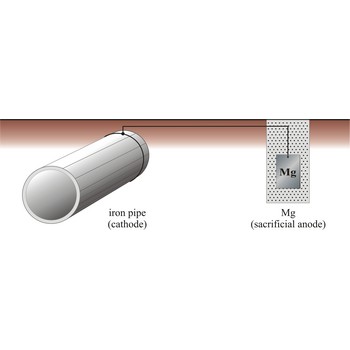
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
convection → konvekcija
Convection is the process by which heat is transferred from one part of a fluid to another by movement of the fluid itself. There are two methods by which this can be carried out.
Natural convection, in which movement occurs as a result of gravity. Heat transferred through a fluid medium, such as air or water, by currents that result from the rising of less dense, warm fluid and the sinking of heavier, cooler fluid.
Forced convection is where hot fluid is transferred from one region to another by a mechanical means (fans or pumps).
irreversible galvanic cell → nepovrativi galvanski članak
Irreversible galvanic cell is a chemical source of direct current, in which reactions that take place on the electrodes are irreversible.
nonelectrolyte → neelektrolit
Nonelectrolytes are substances which in solutions do not dissociate into ions and they do not conduct electric current.
ohm → om
Ohm (Ω) is the SI derived unit of electric resistance. The ohm is the electric resistance between two points of a conductor when a constant difference of potential of one volt, applied between these two points, produces in this conductor a current of one ampere, this conductor not being the source of electromotive force (Ω = V/A). The unit was named after the German physicist Georg Simon Ohm (1789-1854).
overpotential → prenapon
Overpotential (η) is a potential that must be applied in an electrolytic cell in addition to the theoretical potential required to liberate a given substance at an electrode. The value depends on the electrode material and on the current density.
cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
Citing this page:
Generalic, Eni. "SG-003A Voltage Current Signal Generator." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table