Newton’s gravitational law → Newtonov zakon gravitacije
Every object in the universe attracts every other object with a force (gravitational force FG) directed along the line through centres of the two objects that is proportional to the product of their masses and inversely proportional to the square of the distance between them.
m1 and m2 are masses of the two objects and r is the distance between them. G is universal constant of gravitation, which equals 6.67•10-26 N m2 kg-2. Strictly speaking, this law applies only to objects that can be considered pointlike object. Otherwise, the force has to be found by integrating the forces between various mass elements.
It is more properly to express Newton’s gravitational law by vector equation:
in which r1 and r2 are position vectors of masses m1 and m2.
Gravitational forces act on distance. Newton’s gravitational law is derived from Kepler’s law for planetary motion, using a physical assumption considering Sun as the centre and the source of gravitational force.
Additionally, every object moves in the direction of the force acting on it, with acceleration that is inversely proportional to the mass of object. For bodies on the surface of Earth, the distance r in gravitational law formula is practically equal to the Earth radius, RE. If the mass of the body on Earth surface is m and the mass of earth is ME, the gravitational force acting on that body can be expressed as:
where g is gravitational acceleration which is, although dependent on geographical latitude, usually considered as constant equal to 9.81 m s-2.
radiography → radiografija
Radiography is nondestructive method of internal examination in which metal objects are exposed to a beam of X-ray or gamma radiation. Differences in thickness, density, or absorption caused by internal defects or inclusions are apparent in the shadow image produced on a fluorescent screen or photographic film placed behind the object.
Raman effect → Ramanov efekt
Raman effect is a type of scattering of electromagnetic radiation in which light suffers a change in frequency and a change in phase as it passes through a material medium. Named according to the Indian physicist C. V. Raman (1889-1970). The intensity of Raman scattering is about one-thousandth of that in Rayleigh scattering in liquids.
nitrogen → dušik
Nitrogen was discovered by Daniel Rutherford (Scotland) in 1772. The origin of the name comes from the Greek words nitron genes meaning nitre and forming and the Latin word nitrum (nitre is a common name for potassium nitrate, KNO3). It is colourless, odourless, generally inert gas. Minimally reactive at room temperature. A component of many organic and inorganic compounds. Makes up about 78 % of earth’s atmosphere. Nitrogen is obtained from liquid air by fractional distillation. Primarily to produce ammonia and other fertilizers. Also used in making nitric acid, which is used in explosives. Also used in welding and enhanced oil recovery.
nobelium → nobelij
Nobelium was discovered by Nobel Institute of Physics in Stockholm and later by Albert Ghiorso, Torbjorn Sikkeland, J. R. Walton and Glenn T. Seaborg (USA) in 1958. Named in honour of Alfred Nobel, Swedish chemist who discovered dynamite and founder of the Nobel Prizes. It is synthetic radioactive metal. Nobelium was made by bombarding curium with carbon-13.
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
Raoult’s law → Raoultov zakon
Raoult’s law is the expression for the vapour pressure pA of component A in an ideal solution, viz.,
where xA is the mole fraction of component A and pAo the vapour pressure of the pure substance A.
normal conditions → normalni uvjeti
Gas is under normal (or standard) conditions when: p0 = 105 Pa and T0 = 273.15 K (0 °C). IUPAC recommends that the former use of the pressure of 1 atm as standard pressure (equivalent to 101 325 Pa) should be discontinued. At these conditions, the molar volume of gas Vm0 is 0.022 711 m3 (22.711 L).
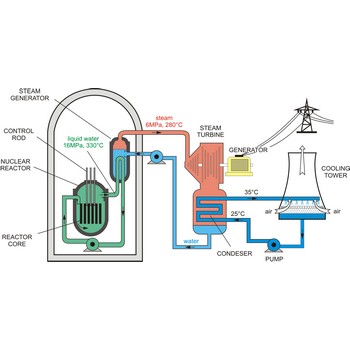
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table