honey → med
Honey is a sweet, amber colored, viscous fluid produced by honeybees from the nectar of flowers. It is composed primarily of fructose (about 40 %), glucose (about 35 %), and water (up to 20 %). In addition, honey contains sucrose, maltose, trisaccharides, and small amounts of minerals, vitamins, and enzymes.
horse power → konjska snaga
Horse power is an obsolete non-SI of power introduced by James Watt in 1782 to allow describing the power of steam machinery. It was equal to the work effort of a horse needed to raise vertically 528 cubic feet of water to one metre in one minute (HP = 735.498750 W).
hygroscopic → higroskopnost
When some substance is hygroscopic, it means that that substance can absorb moisture from air.
entropy → entropija
Entropy (S) is a measure of the unavailability of a system’s energy to do work; in a closed system, an increase in entropy is accompanied by a decrease in energy availability. When a system undergoes a reversible change the entropy (S) changes by an amount equal to the energy (Q) transferred to the system by heat divided by the thermodynamic temperature (T) at which this occurs.
All real processes are to a certain extent irreversible changes and in any closed system an irreversible change is always accompanied by an increase in entropy.
enzyme → enzim
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
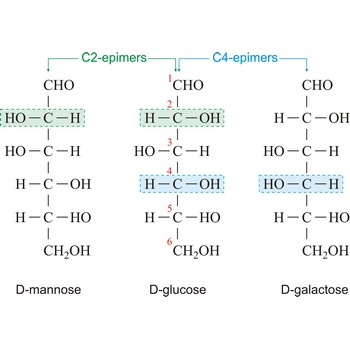
epimer → epimer
Epimers are diastereoisomers that have the opposite configuration at only one of two or more chiral centers present in the respective molecular entities. For example D-glucose and D-mannose, which differ only in the stereochemistry at C-2, are epimers, as are D-glucose and D-galactose (which differ at C-4).
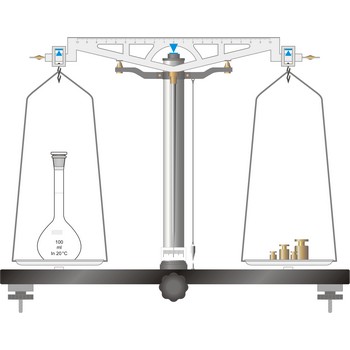
equal-arm balance → vaga s jednakim krakovima
The simplest type of balance, the equal-arm balance, is an application of a first class lever. The beam of the balance is supported on a central knife-edge, usually of agate, which rests upon a plane agate plate. The point of support is called the fulcrum. Two pans of equal weight are suspended from the beam, one at each end, at points equidistant from the fulcrum. A long pointer attached at right angles to the beam at the fulcrum indicates zero on a scale when the beam is at rest parallel to a level surface.
To prevent the knife-edge from becoming dull under the weight of the beam and pans the balance is equipped with a special device called an arrest. The arrest is operated by means of milled knob underneath the base plate in the middle and in front of the balance (sometimes the arrest knob is at one side of the balance).
The object to be weighed is placed on one pan, and standard weights are added to the other until the balance of the beam is established again. When not in use and during loading or unloading of the pans, the balance should be arrested.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
ideal gas → idealni plin
Ideal gas is a gas in which there is complete absence of cohesive forces between the component molecules; the behaviour of such a gas can be predicted accurately by the ideal gas equation through all ranges of temperature and pressure. The concept is theoretical, since no actual gas meets the ideal requirement.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table