formaldehyde → formaldehid
Formaldehyde (methanal) is a colourless gas, HCHO; r.d. 0.815 (at -20 °C); m.p. -92 °C; b.p. -21 °C. It is the simplest aldehyde, made by the catalytic oxidation of methanol (500 °C; silver catalyst) by air. It forms two polymers: methanal trimer and polymethanal.
fouling → obrastanje
1. Fouling is the deposition of insoluble materials, such as bacteria, colloids, oxides and water-borne debris, onto the surface of a reverse osmosis or ultrafiltration membrane. Fouling is associated with decreased flux rates and may also reduce the rejection rates of reverse osmosis membranes. Fouling also refers to the accumulation of normally inorganic deposits on heat exchanger tubing.
2. Fouling is an accumulation of marine organism deposits on a submerged metal surface.
disaccharide → disaharid
Disaccharides are compounds in which two monosaccharides are joined by a glycosidic bond. A glycosidic bond to the anomeric carbon can be either α or β. For example, maltose, the disaccharide obtained by enzyme-catalyzed hydrolysis of starch, consists of two D-glucopyranose units joined by a 1,4’-α-glycoside bond. The "prime" superscript indicates that C-4 is not in the same ring as C-1. Unlike the other disaccharides, sucrose is not a reducing sugar and does not exhibit mutarotation because the glycosidic bond is between the anomeric carbon of glucose and the anomeric carbon of fructose.
fraction → udio
Fraction is a ratio of two quantities of the same kind, the numerator quantity applying to one constituent (or part) of the system and the denominator to the sum of quantities for all constituents (parts) of the system. When applied to mixtures fractions represent a group of three quantities: mass fraction, volume fraction and amount fraction (or mole fraction equal to the number fraction).
fractional crystallisation → frakcijska kristalizacija
Fractional crystallisation is a method of separating a mixture of soluble solids by dissolving them in a suitable hot solvent and then lowering the temperature slowly. The least soluble component will crystallise out first, leaving the other components in the solution. By controlling the temperature, it is sometimes possible to remove each component in turn.
francium → francij
Francium was discovered by Marguerite Perey (France) in 1939. Named for France, the nation of its discovery. It is highly rare and unstable, radioactive metal. Chemical properties similar to cesium. Francium is formed by decay of actinium. Produced by bombarding radium or astatine with neutrons. Since its isotopes have such short half-lives there are no commercially significant compounds of francium.
dissociation constant → konstanta disocijacije
Dissociation constant is a constant whose numerical value depends on the equilibrium between the undissociated and dissociated forms of a molecule. A higher value indicates greater dissociation.
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
The equilibrium constant of such a dissociation is called the acid dissociation constant or acidity constant, given by
The concentration of water [H2O] can be taken as constant.
Similarly, for a base, the equilibrium
is also a dissociation; with the base dissociation constant or basicity constant, given by
Ka (Kb) is a measure of the strength of the acid (base).
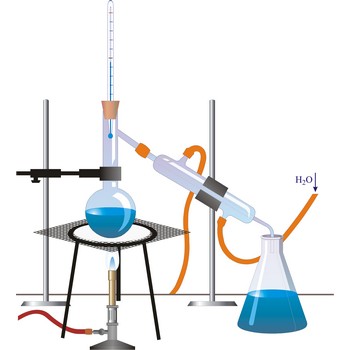
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
freezing point → ledište
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure.
See Melting point
gas thermometer → plinski termometar
Gas thermometer is a device for measuring temperature in which the working fluid is a gas.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table