cobalt → kobalt
Cobalt was discovered by Georg Brandt (Germany) in 1735. The origin of the name comes from the German word kobald meaning goblin or evil spirit. It is hard, ductile, lustrous bluish-grey metal. Surfaces stable in air. Reacts over time with dilute acids. It has remarkable magnetic properties. Cobalt occurs in compounds with arsenic and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a by-product of refining nickel, copper and iron. Used in many hard alloys; for magnets, ceramics and special glasses. Radioactive cobalt-60 is used in cancer therapy.
coenzyme a → koenzim a
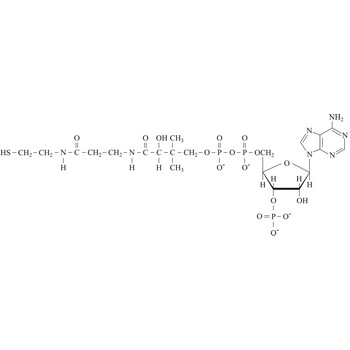
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
covalentity → kovalentnost
Covalentity is a maximum number of covalent bond that one atom can make, it is equal to the number of hydrogen atoms that are combined with other atoms. It is usually constant.
covering power → prekrivna moć
Covering power is the ability of a plating solution under a set of specified plating conditions to deposit metal on the surfaces of recesses or deep holes. This term suggests an ability to cover, but not necessarily to build up, a uniform coating.
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
critical volume → kritični volumen
Critical volume is the volume of a fixed mass of a fluid at critical temperature and pressure.
cryoscopic constant → krioskopska konstanta
Cryoscopic constant (Ef) is the constant that expresses the amount by which the freezing point Tf of a solvent is lowered by a non-dissociating solute, through the relation
where m is the molality of the solute.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table