chemical technology → kemijska tehnologija
Chemical technology is a branch of applied chemistry that concerns technical methods and devices in order to manufacture a chemical product.
chemotherapy → kemoterapija
Chemotherapy is the treatment of disease by means of chemicals that have a specific toxic effect upon the disease producing microorganisms (antibiotics) or that selectively destroy cancerous tissue (anticancer therapy).
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
catalytic cracking → katalitčko krekiranje
Catalytic cracking is a petroleum refining process in which heavy-molecular weight hydrocarbons are broken up into light hydrocarbon molecules by the application of heat and pressure in the presence of a catalyst.
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
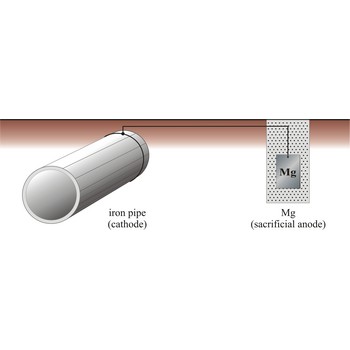
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
chlorofluorocarbons → klorofluorougljici
Chlorofluorocarbons (CFC) are manmade chemicals containing chlorine, fluorine, and carbon. CFCs are used for industrial purposes and in the home for refrigeration, air conditioning, aerosols and as a raw material in polystyrene production.
chlorosity → klorocitet
Chlorosity is the quantity determined by volumetric methods and is defined in the same manner as chlorinity except that the sample unit is 1 L of sea water rather than 1 kg of sea water weighed in vacuo.
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table