bathymetry → batimetrija
Bathymetry (from the Greek word bathus, deep) is the science of measuring the depths of the oceans, seas, lakes, etc. or the topographic maps of the sea floor that result from such measurements. Bathymetric charts are designed to present accurate, measurable description and visual presentation of the submerged terrain.
artificial radioactive isotope → umjetni radioaktivni izotop
Artificial radioactive isotopes are formed when an atom is bombed with an accelerator or exposing it to slow moving neutrons in a nuclear reactor. In this way isotopes (radionuclides) are obtained which are non-existent in nature because of their unstability and radioactive transition into stable isotopes. Most important radioactive isotopes are:
Radioactive isotope of cobalt is formed when ordinary metal cobalt is bombed with neutrons in a nuclear reactor.
Radioactive isotope of phosphorus is formed when ordinary phosphorus is bombed with deuterons produced in cyclotron.
radioactive isotope of carbon is formed when a nitrogen is bombed with slow moving neutrons in a nuclear reactor. It is mostly used as a radioactive indicator.
aspartic acid → asparaginska kiselina
Aspartic acid is an electrically charged amino acids with acidic side chains. As a group the charged amino acids are relatively abundant and are generally located on the surface of the protein. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Aspartic acid (sometimes referred to as asparate depending on pH) is non-essential in mammals, being produced from oxaloacetate by transamination.
- Abbreviations: Asp, D
- IUPAC name: 2-aminobutanedioic acid
- Molecular formula: C4H7NO4
- Molecular weight: 133.10 g/mol
berkelium → berkelij
Berkelium was discovered by Stanley G. Thompson, Albert Ghiorso and Glenn T. Seaborg (USA) in 1949. Named after Berkeley, a city in California, home of the University of California, USA. It is synthetic radioactive metal. Berkelium was made by bombarding americium with alpha particles.
astatine → astat
Astatine was discovered by Emilio Gino Segrè, Dale R. Corson and K. R. MacKenzie (USA) in 1940. The origin of the name comes from the Greek word astatos meaning unstable. It is unstable, radioactive member of the halogen group. Astatine does not occur in nature. Similar to iodine. Produced by bombarding bismuth with alpha particles. Since its isotopes have such short half-lives there are no commercially significant compounds of astatine.
atmosphere → atmosfera
1. Atmosphere is the column of air which is extending several hundred kilometers above the surface the Earth's surface. The density of this air decreases as you proceed up from the surface. The air in the atmosphere consists of 78 % nitrogen, 21 % oxygen, and 0.9 % argon. The remaining 0.1 % of the atmosphere consists of ozone, water vapor, carbon dioxide, methane, helium, and neon. The atmosphere is divided into different regions. The lowest two layers are the troposphere (the layer closest to the earth) and the stratosphere respectively. These two layers contain more than 99 % of the atmospheric molecules.
2. Standard atmosphere (atm) is an obsolete pressure and stress unit which should be discontinued. It is unit of pressure equal to the air pressure measured at mean sea level.
1 atm = 101 325 Pa
Technical atmosphere (at) is an obsolete MKpS pressure and sttress derived unit.
1 at = 98 066.5 Pa
1 atm = 1.033 227 453 at
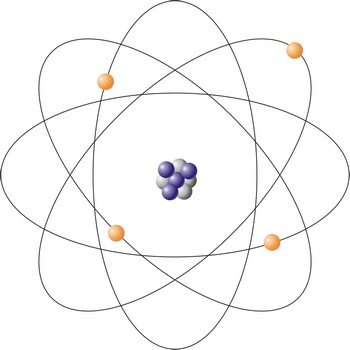
atom → atom
Atom is an atom is the smallest particle of an element that retains the chemical properties of the element. Rutherford-Bohr’s model represents the atom as a positively charged core of a size around 10-14 m composed of protons (positive particles) and neutrons (neutral particles) around which negatively charged electrons circle. The number of protons and electrons are equal, so the atom is an electrically a neutral particle. Diameter of the atom is about 10-10 m.
beta radiation → beta zračenje
Streams of beta particles are known as beta ray or beta radiation. Beta rays may cause skin burns and are harmful within the body. A thin sheet of metal can afford protection to the skin.
bioelement → bioelement
Bioelement is any chemical element that is found in the molecules and compounds that make up living organism.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table