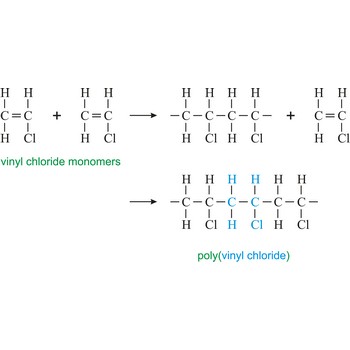
polyvinyl chloride → polivinil klorid
Poly(vinyl chloride) or the PVC is hard and resistant homopolymer produced by the polymerization of the gas vinyl chloride [CH2CHCl]. The pure polymer is hard, brittle and difficult to process, but it becomes flexible when plasticizers are added. After mixing with plasticizers, stabilizers, and pigments, the resin may be fabricated by techniques such as calendering, molding, or extrusion into flexible articles such as raincoats, shower curtains, and packaging films. The resin is not plasticized for use in making rigid products such as water pipe, plumbing fittings, and phonograph records.
potassium → kalij
Potassium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Arabic word qali meaning alkali (the origin of the symbol K comes from the Latin word kalium). It is soft, waxy, silver-white metal. Fresh surface has silvery sheen. Quickly forms dull oxide coating on exposure to air. Reacts strongly with water. Reacts with water to give off flammable gas. Reacts violently with oxidants. Occurs only in compounds. Potassium is found in minerals like carnallite [(KMgCl3)·6H2O] and sylvite (KCL). Pure metal is produced by the reaction of hot potassium chloride and sodium vapours in a special retort. Used as potash in making glass and soap. Also as saltpetre, potassium nitrate (KNO3) to make explosives and to colour fireworks in mauve. Vital to function of nerve and muscle tissues.
potential energy → potencijalna energija
Potential energy (Ep) is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy). Gravitational potential energy is the energy associated with the state of separation between bodies that attracts each other via gravitational force. Elastic potential energy is the energy associated with the state of compression or extension of an elastic object. Thermal energy is associated with the random motions of atoms and molecules in a body.
potentiometric titration → potenciometrijska titracija
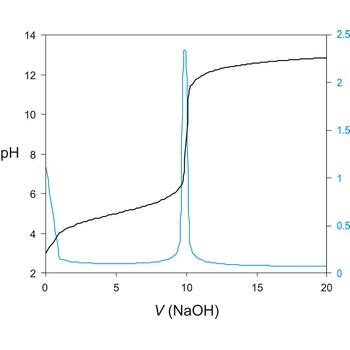
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
system → sustav
System is the region under consideration, as distinguished from the rest of the universe (the environment). Systems may be separated from environments by boundaries that prevent the transfer of mass (a closed system), of heat (an adiabatic system), or of any energy (an isolated system). Systems that exchange mass with the environment are open systems.
temperature → temperatura
Temperature is a measure to the average kinetic energy of its molecules. The SI unit in which thermodynamic temperature is expressed is the kelvin (K).
temperature rating → temperaturno područje
Temperature rating is the maximum and minimum temperature at which the material may be used in continuous operation without loss of its basic properties. For example, temperature ratings are often quoted for electrical insulators, specifying the maximum temperature at which they provide adequate protection against electrical breakdown.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table