Kohlrausch’s law → Kohlrauschov zakon
Kohlrausch’s law states that the equivalent conductivity of an electrolyte at infinite dilution is equal to the sum of the conductances of the anions and cations. If a salt is dissolved in water, the conductivity of the solution is the sum of the conductances of the anions and cations. The law, which depends on the independent migration of ions, was deduced experimentally by the German chemist Friedrich Kohlrausch (1840-1910).
law of conservation of mass → zakon o očuvanju mase
Law of conservation of mass states that no detectable gain or loss in mass occurs in chemical reactions. The state of a substance may change in a chemical reaction, for example, from a solid to a gas, but its total mass will not change. Note that the energy released (exothermic) or adsorbed (endothermic) in a chemical reaction is a result of energy transfer between atoms and their environment.
Nernst’s division law → Nernstov zakon razdjeljenja
Nernst’s division law states that a substance is divided between two solvents in a way that proportion of concentrations of that substance is at certain temperatures constant, under the condition that both solvents are in the same molecular state. Division coefficient is a proportion of substance concentration in solvents A i B at a defined temperature.
Appearance of division is used for substance extraction.
Ostwald’s dilution law → Ostwaldov zakon razrjeđenja
Ostwald’s dilution law is a relation for the concentration dependence of the molar conductivity Λ of an electrolyte solution, viz.
where c is the solute concentration, Kc is the equilibrium constant for dissociation of the solute, and L0 is the conductivity at cΛ = 0. The law was first put forward by the German chemist Wilhelm Ostwald (1853-1932).
Snell’s law → Snellov zakon
When a light ray comes on a boundary between two transparent media, it will be partly reflected and partly refracted. Both rays, reflected and refracted ray, lay in the plane of incidence. The angle of reflection is equal to the angle of incidence. The angle of refraction (Θ2) is related to the angle of incidence (Θ1) via Snell’s law:
where n1 and n2 are dimensionless constants - indexes of refraction of the two media.
Archimedes law → Arhimedov zakon
The upward force (buoyancy force) is exerted on a body floating in a fluid. It equals the weight of the displaced fluid.
Coulomb’s law → Coulombov zakon
Coulomb’s law is the statement that the force F between two electrical charges q1 and q2 separated by a distance r is
where εo is the permittivity of a vacuum, equal to
Raoult’s law → Raoultov zakon
Raoult’s law is the expression for the vapour pressure pA of component A in an ideal solution, viz.,
where xA is the mole fraction of component A and pAo the vapour pressure of the pure substance A.
gravitational constant → gravitacijska konstanta
Gravitational constant (G) is the universal constant in the equation for the gravitational force between two particles
where r is the distance between the particles and m1 and m2 are their masses.
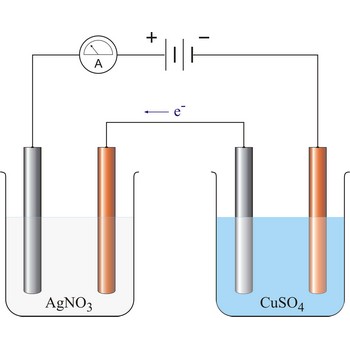
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
Citing this page:
Generalic, Eni. "Newtonov zakon gravitacije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table