electron configuration → elektronska konfiguracija
The electron configuration shows how many electrons there are in an atom or ion and their distribution along orbitals (see Table of electronic configuration of elements). Structure and all regularity in the periodic system depend upon electronic configuration of atoms of elements. Characteristics of elements mainly depend on electronic configuration of the outer shell. Refilling of the new electronic shell atoms of elements of similar electronic configuration emerge as well as in the previous shell, which adds up to periodicities of characteristics of elements.
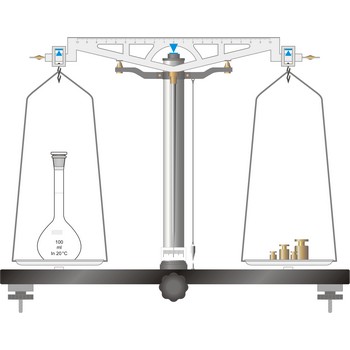
equal-arm balance → vaga s jednakim krakovima
The simplest type of balance, the equal-arm balance, is an application of a first class lever. The beam of the balance is supported on a central knife-edge, usually of agate, which rests upon a plane agate plate. The point of support is called the fulcrum. Two pans of equal weight are suspended from the beam, one at each end, at points equidistant from the fulcrum. A long pointer attached at right angles to the beam at the fulcrum indicates zero on a scale when the beam is at rest parallel to a level surface.
To prevent the knife-edge from becoming dull under the weight of the beam and pans the balance is equipped with a special device called an arrest. The arrest is operated by means of milled knob underneath the base plate in the middle and in front of the balance (sometimes the arrest knob is at one side of the balance).
The object to be weighed is placed on one pan, and standard weights are added to the other until the balance of the beam is established again. When not in use and during loading or unloading of the pans, the balance should be arrested.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
ideal gas law → jednadžba stanja idealnog plina
The generalized ideal gas law is derived from a combination of the laws of Boyle and Charles. Ideal gas law is the equation of state
which defines an ideal gas, where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
ideal solution → idealna otopina
Ideal solution is a solution in which solvent-solvent and solvent-solute interactions are identical, so that properties such as volume and enthalpy are exactly additive. Ideal solutions follow Raoult’s law, which states that the vapour pressure pi of component i is pi = xi pi*, where xi is the mole fraction of component i and pi* the vapour pressure of the pure substance i.
Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
inertial reference frames → inercijski referentni sustavi
When two frames of reference are moving relative to each other at constant velocity, they are said to be inertial reference frames. The observers from two such inertial frames measure, in general, different velocities of a moving particle. On the other hand, they measure the same acceleration for the particle. The laws of physics must have the same form in all inertial reference frames (the principle of invariance).
spectrophotometer → spektrofotometar
Spectrophotometer is an instrument for measuring the amount of light absorbed by a sample.
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
standard electrode potential → standardni elektrodni potencijal
Standard electrode potential (E°) (standard reduction potentials) are defined by measuring the potential relative to a standard hydrogen electrode using 1 mol solution at 25 °C. The convention is to designate the cell so that the oxidised form is written first. For example,
The e.m.f. of this cell is -0.76 V and the standard electrode potential of the Zn2+|Zn half cell is -0.76 V.
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
Citing this page:
Generalic, Eni. "Nernstov zakon razdjeljenja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table