reversible process → reverzibilan proces
Reversible process or reaction is those that can be reversed by an infinitesimally small change in conditions. For example, ice and water coexist at 101 325 Pa and 0 °C; a very slight temperature increase causes the ice to melt; a tiny temperature decrease causes the water to freeze. Melting or freezing under these conditions can be considered reversible.
weak acid → slaba kiselina
Weak acid is an acid that incompletely dissociated in aqueous solution. Acetic acid is an example of a weak acid
weak base → slaba baza
Weak base is a base that only partially dissociates into ions in solution. Weak bases are weak electrolytes. Ammonia is an example of a weak base
silver → srebro
Silver has been known since ancient times. The origin of the name comes from the Latin word argentum meaning silver. It is silvery-ductile and malleable metal. Stable in water and oxygen. Reacts with sulfur compounds to form black sulfides. Silver is found in ores called argentite (AgS), light ruby silver (Ag3AsS3), dark ruby silver (Ag3SbS3) and brittle silver. Used in alloys for jewellery and in other compounds for photography. It is also a good conductor, but expensive.
sodium → natrij
Sodium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Latin word natrium meaning sodium carbonate. It is soft silvery-white metal. Fresh surfaces oxidize rapidly. Reacts vigorously, even violently with water. Reacts with water to give off flammable gas. Burns in air with a brilliant white flame. Sodium is obtained by electrolysis of melted sodium chloride (salt), borax and cryolite. Metallic sodium is vital in the manufacture of organic compounds. Sodium chloride (NaCl) is table salt. Liquid sodium is used to cool nuclear reactors.
stoichiometric coefficient → stehiometrijski koeficijent
Stoichiometric coefficient (ν) is the number appearing before the symbol for each compound in the equation for a chemical reaction. By convention, it is negative for reactants and positive for products.
Stoichiometric coefficients describe the stoichiometry of the chemical reaction.
In this equation, a, b, c and d are called as Stoichiometric coefficients of the A, B, C and D respectively.
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
Solvay’s process → Solvayev postupak
Solvay ’s process is an industrial process for producing sodium carbonate from sodium chloride and ammonia and carbon dioxide.
Carbon dioixide is produced by the thermal decomposition of limestone, CaCO3(s).
Quicklime, formed as a by-product of the thermal decomposition of limestone, is treated with water to form calcium hydroxide.
Calcium hydroxide is heated with ammonium chloride to form ammonia and calcium chloride (by product).
Carbon dioxide reacts with ammonia to form ammonium carbonate.
Ammonium carbonate further reacts with carbon dioxide to form ammonium bicarbonate.
Ammonium bicarbonate then react with sodium chloride to form sodium bicarbonate.
Dry sodium bicarbonate is heated in rotary furnace to give anhydrous sodium carbonate or soda ash.
The carbon dioxide produced is recycled back into the process.
strontium → stroncij
Strontium was discovered by Sir Humphry Davy (England) in 1808. Named after the village of Strontian in Scotland. It is soft, malleable, silvery-yellow metal. Combustible in air, will react with water. Exposed surfaces form protective oxide film. Metal ignites and burns readily. Strontium is found in minerals celestite and strontianite. Used in flares and fireworks for crimson colour. Strontium-90 is a long lived highly radioactive fallout product of atomic-bomb explosions.
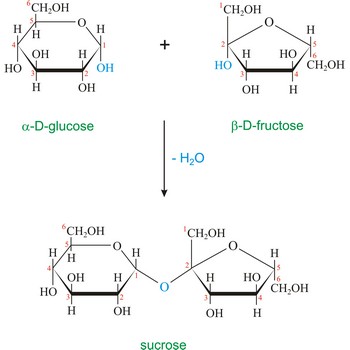
sucrose → saharoza
Sucrose (saccharose), or ordinary table sugar, is a disaccharide in which α-D-glucopyranose and β-D-fructofuranose are joined at their anomeric carbons by a glycosidic bond. There are no hemiacetals remaining in the sucrose and therefore sucrose is not a reducing sugar and does not exhibit mutarotation. Sugar is a white crystalline sweet compound found in many plants and extracted from sugar cane and sugar beet. It is used as a sweetening agent in food and drinks. If heated to 200 °C, sucrose becomes caramel. When sucrose is hydrolyzed it forms an equimolar mixture of glucose and fructose. This mixture of monosaccharides is called invert sugar. Honeybees have enzymes called invertases that catalyze the hydrolysis of sucrose. Honey, in fact, is primarily a mixture of glucose, fructose, and sucrose.
Citing this page:
Generalic, Eni. "Millonova reakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table