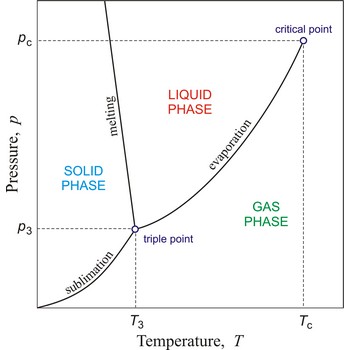
critical point → kritična točka
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
critical pressure → kritični tlak
Critical pressure is the pressure of a fluid in its critical point; i.e. when it is at its critical temperature and critical volume.
critical temperature → kritična temperatura
Critical temperature is the temperature of the liquid-vapour critical point, that is, the temperature above which a gas cannot be liquefied by an increase of pressure.
homogenic reaction → homogena reakcija
Homogenic reactions are those reactions in which products and reactants are in the same phase (aggregate state).
cryogenic fractionation → kriogena frakcinacija
Cryogenic fractionation is a process of separation of gases by cooling them until they enter their liquid state. Large scale gas production companies use this method to produce liquid oxygen, liquid nitrogen etc. Gases have different boiling points (the temperature at which they change from liquid to gas). Oxygen has a boiling point of -183 °C, and nitrogen a boiling point of -195.8 °C. Therefore by cooling the gas mixture to -183 °C, the oxygen can be collected as liquid and the nitrogen remains its gaseous form.
Curie → Curie
Maria Sklodowska-Curie (1867-1934) Polish-born French chemist who went to Paris in 1891. She married the physicist Pierre Curie (1859-1906) in 1985 and soon began work on seeking radioactive elements other than uranium in pitchblende (to account for its unexpectedly high radioactivity). By 1898 she had discovered radium and polonium although it took her years to purify them. In 1903 the Curies shared the Nobel Prize for physics with Henri Becquerel, who had discovered radioactivity.
differential thermal analysis → diferencijalna termalna analiza
Differential thermal analysis (DTA) is a technique that is often used to analyze materials that react or decompose at higher temperatures. The difference in temperature between the sample and an inert reference material is monitored as both are heated in a furnace. Phase transitions and chemical reactions taking place in the sample on heating cause the temperature difference to become larger, at temperatures that are characteristic of the sample.
diffusion → difuzija
Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. The rate of diffusion is proportional to the concentration of the substance and increases with temperature. The theoretical principles are stated in Fick’s laws.
Citing this page:
Generalic, Eni. "Gibbsov zakon faza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table