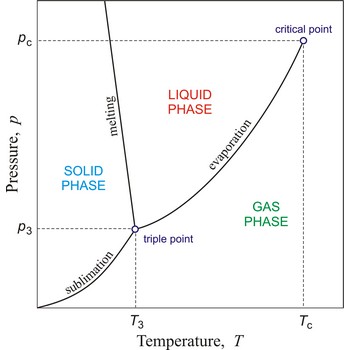
critical point → kritična točka
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
flash point → temperatura zapaljenja
Flash point is the lowest temperature at which a liquid or volatile solid gives off vapour sufficient to form an ignitable mixture with the air near the surface of the liquid or within the test vessel (NFPA).
fluid mechanics → mehanika fluida
Fluid mechanics is the study of various properties of the fluid (liquids and gases): velocity, pressure, density and temperature, as functions of space and time.
fractional crystallisation → frakcijska kristalizacija
Fractional crystallisation is a method of separating a mixture of soluble solids by dissolving them in a suitable hot solvent and then lowering the temperature slowly. The least soluble component will crystallise out first, leaving the other components in the solution. By controlling the temperature, it is sometimes possible to remove each component in turn.
critical pressure → kritični tlak
Critical pressure is the pressure of a fluid in its critical point; i.e. when it is at its critical temperature and critical volume.
crucible → lončić za žarenje
Crucible is used for heating small amounts of solid in an oven to very high temperatures. Crucibles are usually made out of porcelain, platinum, nickel or iron.
cryogenic fluid → kriogenski fluid
Cryogenic fluids are used for accessing low temperatures, usually below -150 °C. Cryogenic temperatures are achieved by the rapid evaporation of volatile liquids. The most common laboratory cryogenic fluids are liquid nitrogen (-196 °C). Nitrogen gas is colorless and odorless. The cloud formed when pouring liquid nitrogen is condensed water vapour from the air, not nitrogen gas.
cryogenic fractionation → kriogena frakcinacija
Cryogenic fractionation is a process of separation of gases by cooling them until they enter their liquid state. Large scale gas production companies use this method to produce liquid oxygen, liquid nitrogen etc. Gases have different boiling points (the temperature at which they change from liquid to gas). Oxygen has a boiling point of -183 °C, and nitrogen a boiling point of -195.8 °C. Therefore by cooling the gas mixture to -183 °C, the oxygen can be collected as liquid and the nitrogen remains its gaseous form.
freezing → smrzavanje
Freezing is the change of a liquid into a solid state as the temperature decreases. For water, the freezing point is 0 °C (or 273.16 K).
Citing this page:
Generalic, Eni. "GL3523T Temp." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. 10 Apr. 2025. <https://glossary.periodni.com>.
Glossary
Periodic Table