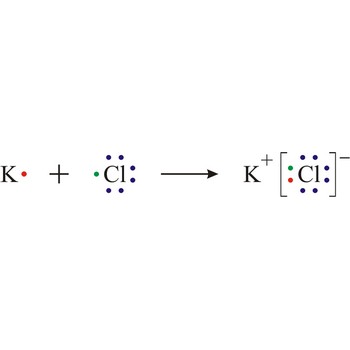substitution → supstitucija
Substitution (substitution reaction) is a reaction in which an atom or fragment within a molecule is replaced with another atom or a fragment.
tertiary alcohol → tercijarni alkohol
Tertiary alcohols are aliphatic alcohols in which the hydroxyl group (-OH) is attached to a tertiary carbon atom.
transuranium element → trasuranski element
Transuranium elements are elements with an atomic number higher than 92 (uranium’s atomic number). Transuranium elements are unstable and occur in extremely low concentrations in nature. Most are made artificially.
ionisation → ionizacija
Ionisation is the process of producing ions. Certain molecules ionise in a solution; for example, acids ionise when dissolved in water.
Electron transfer also causes ionisation in certain reactions, for example sodium and chlorine react by transfer of a valence electron from the sodium atom to the chlorine atom to form the ions that constitute a sodium chloride crystal.
law of conservation of mass → zakon o očuvanju mase
Law of conservation of mass states that no detectable gain or loss in mass occurs in chemical reactions. The state of a substance may change in a chemical reaction, for example, from a solid to a gas, but its total mass will not change. Note that the energy released (exothermic) or adsorbed (endothermic) in a chemical reaction is a result of energy transfer between atoms and their environment.
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
valence bond → valentna veza
In the valence bond theory, a valence bond is a chemical bond formed by overlap of half-filled atomic orbitals on two different atoms.
Citing this page:
Generalic, Eni. "Chaotic Atoms (Dramatic)." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table