dialysis → dijaliza
Dialysis is a method by which large molecules (such as starch or protein) and small molecules (such as glucose or amino acids) may be separated in a solution by selective diffusion through a semipermeable membrane. Through this kind of membrane dissolved particles pass and colloid dimension particles fall behind. For example, if a mixed solution of starch and glucose is placed in a closed container made of a semipermeable substance (such as cellophane), which is then immersed in a beaker of water, the smaller glucose molecules will pass trough the membrane into the water, while the starch molecules remain behind.
Millon’s reaction → Millonova reakcija
Millon’s reaction is used for testing proteins by the appearance of red colour which the proteins give by reacting with a solution of mercury in nitric acid.
fossil fuel → fosilno gorivo
Fossil fuels (coal, oil, and natural gas) are the fuels used by man as a source of energy. They are formed from the remains of living organisms and all have a high carbon or hydrogen content. They have value as fuels on the exothermic oxidation of carbon to form carbon dioxide
and the oxidation of hydrogen to form water
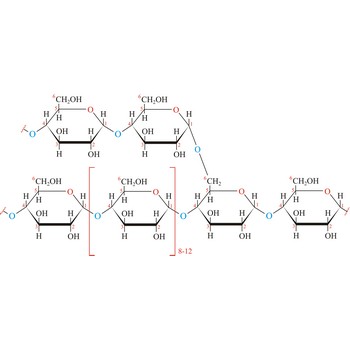
glycogen → glikogen
Glycogen (animal starch) is a polysaccharide that serves the same energy storage function in animals that starch serves in plants. Dietary carbohydrates not needed for immediate energy are converted by the body to glycogen for long term storage (principally in muscle and liver cells). Like amylopectin found in starch, glycogen is a polymer of α(1→4)-linked subunits of glucose, with α(1→6)-linked branches. Glycogen molecules are larger than those of amylopectin (up to 100 000 glucose units) and contain even more branches. Branch points occur about every 10 residues in glycogen and about every 25 residues in amylopectin. The branching also creates lots of ends for enzyme attack and provides for rapid release of glucose when it is needed.
Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
Nessler’s reagent → Nesslerov reagens
Nessler’s reagent is a solution of mercury(II) iodide (HgI2) in potassium iodide and potassium hydroxide named after the German chemist Julius Nessler (1827-1905). It is used in testing for ammonia, with which it forms a brown coloration or precipitate.
organoleptic testing → organoleptičko ispitivanje
Organoleptic testing of matter is a testing of matter and material using the senses of sight, smell, taste and touch, used usually when buying groceries.
Citing this page:
Generalic, Eni. "živo vapno." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table