buffer capacity → puferski kapacitet
Buffer capacity is number of moles of a strong acid or a strong base needed to change pH of 1 dm3 of buffer solution for pH unit.
calorimetry → kalorimetrija
Calorimetry is a measurement of the amount of heat evolved or absorbed in a chemical reaction, change of state, or formation of a solution, or any other event that includes heat transfer.
carbon fibre → ugljično vlakno
Carbon fibres are threadlike strands of pure carbon that are strong and flexible. Carbon fibres can be bound in a plastic resin matrix to form a strong composite. It is light-weight and stronger than steel.
cation exchange → kationski izmjenjivač
Cation exchange is a cationic resin has positive ions built into its structure and therefore exchanges negative ions. In the cation exchange, the side groups are ionised acidic groups, such as (-SO3H, -COOH, -OH) to which cations H+ are attached. The exchange reaction is one in which different cations in the solution displace the H+ from the solid.
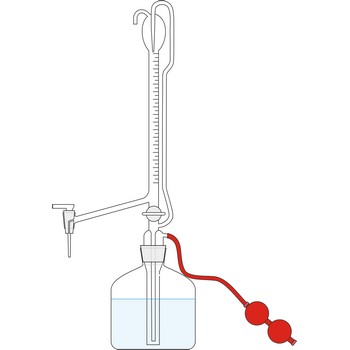
automatic burette → automatska bireta
Automatic burette is used for series of tests. It is connected with a bottle which contains the titration solution. The air is pumped into the bottle by a small rubber air pump, created the pressure in the bottle which the rises the solution to the top of burette. When the the burette is full, the valve is released, the pressure in the bottle falls and the burette automatically sets itself to zero. Work with automatic burettes is by far faster and the consumption of standard solution is smaller.
base → baza
Historically, base is a substance that yields an OH - ion when it dissociates in solution, resulting in a pH>7. In the Brønsted definition, a base is a substance capable of accepting a proton in any type of reaction. The more general definition, due to G.N. Lewis, classifies any chemical species capable of donating an electron pair as a base. Typically, bases are metal oxides, hydroxides, or compounds (such as ammonia) that give hydroxide ions in aqueous solution.
cementation → cementacija
Cementation is any metallurgical process in which the surfaces of a metal is impregnated by some other substance, especially an obsolete process for making steel by heating bars of wrought iron to red heat for several days in a bed of charcoal.
colligative properties → koligativna svojstva
Colligative properties are properties which affect a solvent based on the number of molecules of solute present such as melting point, boiling point and osmotic pressure.
colloid silver → koloidno srebro
Colloid silver is a bright blue-green powder which dissolved in water gives colloid solution of red colour.
colorimeter → kolorimetar
Colorimeter is an instrument used to measure the strength of colorification in a solution.
Citing this page:
Generalic, Eni. "čvrste otopine." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table