conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
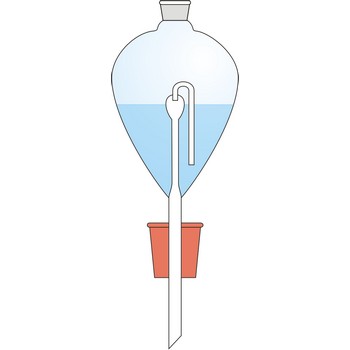
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
heat of sublimation → toplina sublimacije
Heat of sublimation or enthalpy of sublimation is the energy required to convert one mole of a substance from the solid to the gas state (sublimation) without the appearance of the liquid state.
ideal gas → idealni plin
Ideal gas is a gas in which there is complete absence of cohesive forces between the component molecules; the behaviour of such a gas can be predicted accurately by the ideal gas equation through all ranges of temperature and pressure. The concept is theoretical, since no actual gas meets the ideal requirement.
immersion plating → galvaniziranje uranjanjem
Immersion plating involves depositing a metallic coating on a metal immersed in a liquid solution, without the aid of an external electric current. Also called dip plating.
crucible → lončić za žarenje
Crucible is used for heating small amounts of solid in an oven to very high temperatures. Crucibles are usually made out of porcelain, platinum, nickel or iron.
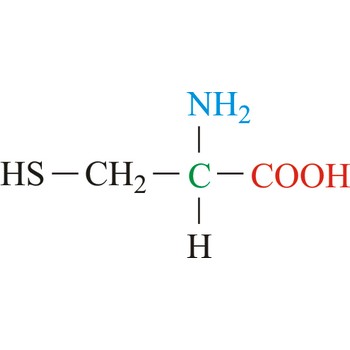
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
dialysis → dijaliza
Dialysis is a method by which large molecules (such as starch or protein) and small molecules (such as glucose or amino acids) may be separated in a solution by selective diffusion through a semipermeable membrane. Through this kind of membrane dissolved particles pass and colloid dimension particles fall behind. For example, if a mixed solution of starch and glucose is placed in a closed container made of a semipermeable substance (such as cellophane), which is then immersed in a beaker of water, the smaller glucose molecules will pass trough the membrane into the water, while the starch molecules remain behind.
insulator → izolator
Insulator is a material in which the highest occupied energy band (valence band) is completely filled with electrons, while the next higher band (conduction band) is empty. Solids with an energy gap of 5 eV or more are generally considered as insulators at room temperature. Their conductivity is less than 10-6 S/m and increases with temperature.
Citing this page:
Generalic, Eni. "čvrste otopine." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table