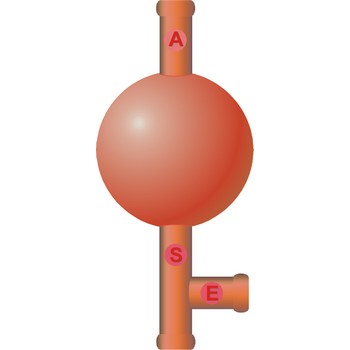
three way safety bulb → propipeta
Three way safety bulb (pipette filler bulb) is used for pipeting. The attachment is placed over the mouth of the pipet. Squeeze the air valve (A) and the bulb simultaneously to empty the bulb of air. Place the tip of the pipet below the solution's surface in the beaker. Gradually squeeze the suction valve (S) to draw liquid into the pipet. If the level of the solution is not high enough, squeeze the air valve (A) and the bulb again to expel the air from the bulb. Draw up more liquid by squeezing the suction valve (S). When the liquid is above the specified volume, stop squeezing the suction valve (S). Do not remove the bulb from the pipet. Do not allow liquid to enter the pipet bulb.
photoelectric threshold → fotoelektrični prag
Photoelectric threshold is a maximum length of electromagnetic wave which can still cause the photoelectric effect.
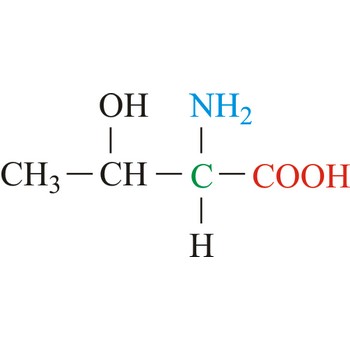
threonine → treonin
Threonine is neutral amino acids with polar side chains. It differs from serine by having a methyl substituent in place of one of the hydrogens on the β carbon. Threonine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Thr, T
- IUPAC name: 2-amino-3-hydroxybutanoic acid
- Molecular formula: C4H9NO3
- Molecular weight: 119.12 g/mol
Allihn condenser → Allihnovo hladilo
Allihn condenser or bulb condenser consists of an outer water jacket and the inner glass tube with a series of spherical bubbles to maximize the thermal contact with the cooling water. It is named after its inventor, the German chemist Felix Richard Allihn (1854-1915).
argon → argon
Argon was discovered by Lord Raleigh and Sir William Ramsay (Scotland) in 1894. The origin of the name comes from the Greek word argos meaning inactive. It is colourless and odourless noble gas. Chemically inert. It is the third most abundant element in the earth’s atmosphere and makes up about 1 %. Argon is continuously released into the air by decay of radioactive potassium-40. Pure form is obtained from fractional distillation of liquid air. Used in lighting products. It is often used in filling incandescent light bulbs. Some is mixed with krypton in fluorescent lamps. Crystals in the semiconductor industry are grown in argon atmospheres.
Arrhenius equation → Arheniusova jednadžba
In 1889, Svante Arrhenius explained the variation of rate constants with temperature for several elementary reactions using the relationship
where the rate constant k is the total frequency of collisions between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy Ea at a temperature of T (in kelvin). R is the universal gas constant.
asbestos → azbest
Asbestos is any one of a group of fibrous amphibole minerals (from the silicate group). It has widespread commercial uses because of its resistance to heat, chemical inertness., and high electrical resistance. Since 1970s short asbestos fibres have been recognized as a cause of asbestosis, a serious lung disorder, and mesothelioma, a fatal form of lung cancer. These concerns have limited its use and imposed many safety procedures when it is used.
Bunsen burner → Bunsenov plamenik
Bunsen burner is a standard source of heat in the laboratory. German chemist Roberts Bunsen (1811-1899) improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. The Bunsen burner has a vertical metal tube through which a fine jet of fuel gas is directed. Air is drawn in through airholes near the base of the tube and the mixture is ignited and burns at the tube’s upper opening. The flow of this air is controlled by an adjustable collar on the side of the metal tube. When the whole is closed a yellow safety flame is displayed. Where as when the whole is open it displays a power dull blue flame with a faint blue outer flame with a vibrant blue core used u for combustion and hearting. The flame can reach temperatures of 1 500 °C.
carbon fibre → ugljično vlakno
Carbon fibres are threadlike strands of pure carbon that are strong and flexible. Carbon fibres can be bound in a plastic resin matrix to form a strong composite. It is light-weight and stronger than steel.
Citing this page:
Generalic, Eni. "Three-way safety bulb." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table