unsaturated hydrocarbon → nezasićeni ugljikovodik
Unsaturated hydrocarbons are organic compounds containing double (alkenes) or triple (alkynes) bonds in their molecules.
unsaturated solution → nezasićena otopina
Unsaturated solution is a solution that contains less than the maximum possible equilibrium concentration of a solute.
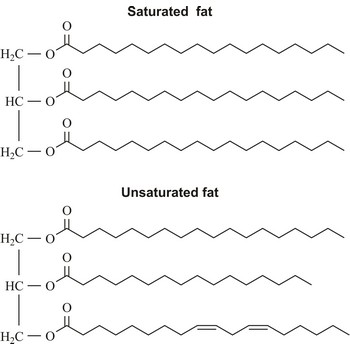
unsaturated fat → nezasićena mast
Unsaturated fats, which include one or more unsaturated fatty acid, are liquid at room temperature (oil) and come from plant oils such as olive, peanut, corn, sunflower, safflower, and soybean. The fish oils may also be high in unsaturated fatty acids.
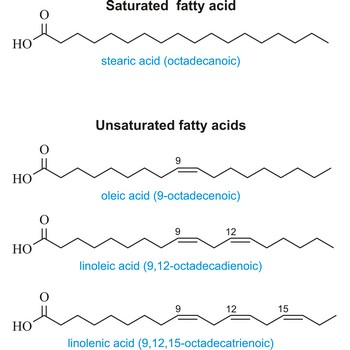
unsaturated fatty acid → nezasićena masna kiselina
Unsaturated fatty acid is a fatty acid whose carbon chain can absorb additional hydrogen atoms. Their carbon chain has one or more double or triple valence bond per molecule. The most important of these are:
| Oleic (9-octadecenoic acid) | CH3(CH2)7CH=CH(CH2)7COOH |
| Linoleic (9,12-octadecadienoic acid) | CH3(CHCH2)3(CH2CH=CH)2(CHCH2)7COOH |
| Linolenic (9,12,15-octadecatrienoic acid) | CH3(CH2CH=CH)3(CHCH2)7COOH |
catalytic hydrogenation → katalitičko hidrogeniranje
Catalytic hydrogenation is the infusing of unsaturated or impure hydrocarbons with hydrogen gas at controlled temperatures and pressures and in the presence of a catalyst for the purpose of obtaining saturated hydrocarbons and/or removing various impurities such as sulphur and nitrogen.
aromatic compounds → aromatski ugljikovodici
Aromatic compounds are a major group of unsaturated cyclic hydrocarbons containing one or more rings, typified by benzene, which has a 6-carbon ring containing three double bonds. All the bonds in benzene (C6H6) are the same length intermediate between double and single C-C bonds. The properties arise because the electrons in the p-orbitals are delocalised over the ring, giving extra stabilization energy of 150 kJ/mol over the energy of Kekulé structure. Aromatic compounds are unsaturated compounds, yet they do not easily partake in addition reactions.
Historical use of the term implies a ring containing only carbon (e.g., benzene, naphthalene), but it is often generalized to include heterocyclic structures such as pyridine and thiophene.
benzene → benzen
Benzene is a colourless liquid hydrocarbon, C6H6, b.p. 80 °C. It is now made from petroleum by catalytic reforming (formerly obtained from coal tar). Benzene is the archetypal aromatic compound. It has an unsaturated molecule, yet will not readily undergo addition reactions. On the other hand, it does undergo substitution reactions in which hydrogen atoms are replaced by other atoms or groups.
In 1865, Friedrich August Kekulé purposed the benzene molecule structure as a hexagonal ring which consists of six carbon atoms with alternate carbon-carbon single and carbon-carbon double bond. But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical and intermediate between those for a single and double bond. The π-orbitals from each neighbouring carbon atom overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity. That is the reason the structural formula of benzene represents as a hexagon with a circle in the center which represents the delocalized electrons.
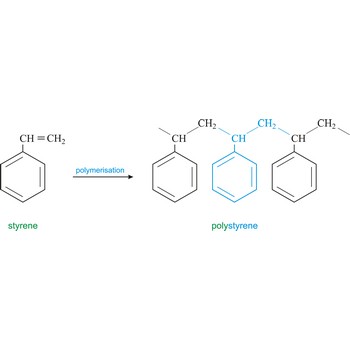
styrene → stiren
Styrene is an unsaturated hydrocarbon (C6H5OC2H3O) colourless, toxic liquid with a strong aromatic aroma. It is soluble in alcohol, ether, acetone, and carbon disulfide, but dissolves only slightly in water. It is used to make plastics such as polystyrene, ABS, styrene-butadiene rubber styrene-butadiene latex and unsaturated polyesters.
Citing this page:
Generalic, Eni. "Unsaturated hydrocarbon." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table