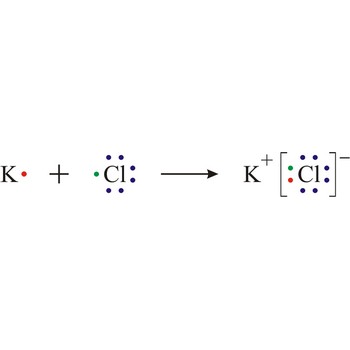cubic close-packed structure → kubična gusta slagalina
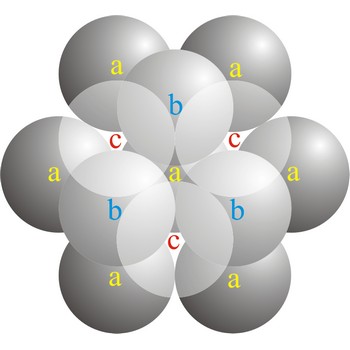
In a cubic close-packed (ccp) arrangement of atoms, the unit cell consists of four layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The atoms in the second layer (b) fit into depressions in the first layer. The atoms in the third layer (c) occupy a different set of depressions than those in the first. The cubic close packed structure can be made by piling layers in the a-b-c-a-b-c-a-b-c... sequence.
close-packed structure → gusta slagalina
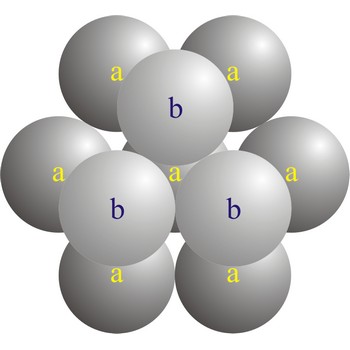
Close packing is the packing of spheres so as to occupy the minimum amount of space. The name close packed refers to the packing efficiency of 74.05 %. There are two types of close packing: hexagonal and cubic. One layer, with atoms centered on sites labeled a. Two layers, with the atoms of the second layer centered on sites labeled b. The third layer can be placed on the sites labeled c (giving cubic close-packing) or over those marked a (giving hexagonal close-packing).
hexagonal close-packed structure → heksagonska gusta slagalina
In a hexagonal close-packed (hcp) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The middle layer (b) contains three atoms nestled between the atoms of the top and bottom layers, hence, the name close-packed. The hexagonal close packed structure can be made by piling layers in the a-b-a-b-a-b... sequence.
body-centered cubic lattice → prostorno centrirana kubična rešetka
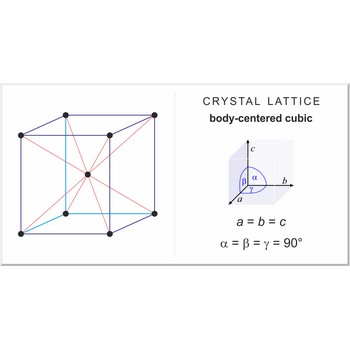
Body-centered cubic lattice (bcc or cubic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a = b = c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the bcc structures the spheres fill 68 % of the volume. The number of atoms in a unit cell is two (8 × 1/8 + 1 = 2). There are 23 metals that have the bcc lattice.
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
simple cubic lattice → jednostavna kubična rešetka
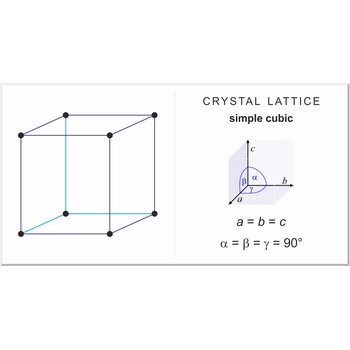
Simple or primitive cubic lattice (sc or cubic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a = b = c and interaxial angels α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the sc structures the spheres fill 52 % of the volume. The number of atoms in a unit cell is one (8×1/8 = 1). This is only one metal (α-polonium) that have the sc lattice.
cubic crystal system → kubični kristalni sustav
Cubic crystal system is also known as the isometric system. The Isometric crystal system characterizes itself by its three equivalent crystallographic axes perpendicular to each other.
a = b = c
α = β = γ = 90°
primary structure of proteins → primarna struktura proteina
Primary structure of proteins is a sequence of defined amino acids in some protein.
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
planary structure → planarna struktura molekule
Planary structure of molecule is a structure of molecule in which all atoms in the molecule lie in the same plane.
Citing this page:
Generalic, Eni. "Cubic close-packed structure." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table