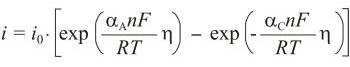Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
Heyrovsky-Ilkovic equation → Heyrovsky-Ilkovičeva jednadžba
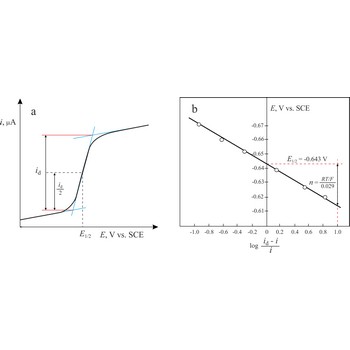
The Heyrovsky-Ilkovic equation describes the entire current-potential curve (polarographic wave) of a reversible redox system in polarography
where R is the gas constant, T is the absolute temperature, F is the Faraday constant, n denotes the number of electrons taking part in the electrode reaction. E1/2 is a unique potential (for a given reaction and supporting electrolyte) termed the half-wave potential.
In order to obtain E1/2 from the above equation, we plot a graph of ln[(id-i)/i] against E. The intercept on the x-axis gives then an accurate value of E1/2. The slope of the obtained straight line is equal to nF/RT from which n is determined.
Boltzmann equation → Boltzmannova jednadžba
Boltzmann equation is a statistical definition of entropy, given by
where S and k are the entropy and Boltzmann’s constant, respectively, and W is the probability of finding the system in a particular state.
chemical equation equalization → izjednačavanje kemijske jednadžbe
Chemical equation equalization is determining values of stechiometric coefficients of reactants and products in a chemical equation in a way that the number of atoms of each element is equal before and after the reaction.
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
complete ionic equation → potpuna ionska jednadžba
Complete ionic equation is a balanced equation that describes a reaction occurring in a solution, in which all strong electrolytes are written as dissociated ions.
equation of state → jednadžba stanja
Equation of state is an equation relating the pressure, volume, and temperature of a substance or system. Equation of state for ideal gas
where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
chemical equation → kemijska jednadžba
Chemical equation is a way of denoting a chemical reaction using the symbol for the participating particles (atoms, molecules, ions, etc.); for example,
The single arrow is used for an irreversible reaction; double arrows are used for reversible reactions. When reactions involve different phases, it is usual to put the phase in brackets after the symbol.
| s | = | solid |
| l | = | liquid |
| g | = | gas |
| aq | = | aqueous |
The numbers a, b, c, and d, showing the relative numbers of molecules reacting, are called the stoichiometric coefficients. The convention is that stoichiometric coefficients are positive for reactants and negative for products. If the sum of the coefficients is zero, the equation is balanced.
rate equation → jednadžba brzine reakcije
Rate equation is an equation that describes the dependence of reaction rate on concentrations of reacting species. It always has the form
where a and b are usually integral exponents.
chemical reaction equation → jednadžba kemijske reakcije
Chemical reactions are symbolically shown with chemical equations. On the left side of the equation we write formulas or substance symbols which enter the chemical reaction, reactants. On the right side formulas or substance symbols which emerge from the chemical reaction, products, are writen.
Each chemical reaction leads to an equilibrium which is moved more or less to one side (left or right). Because of that, in reversible reactions instead of = sign two opposite arrows are put
In order to write down certain chemical reaction equation all reactants and all products and their stechiometric proportions must be known. (See Chemical reaction balancing)
Citing this page:
Generalic, Eni. "Ilkovic equation." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table